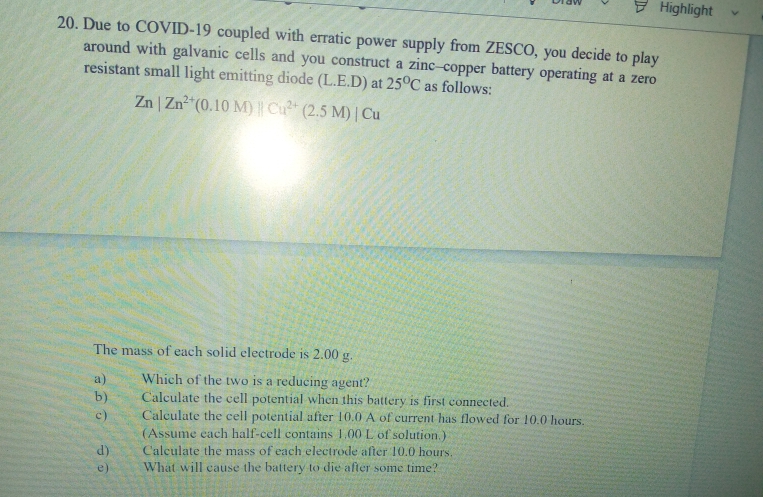
20. Due to COVID-19 coupled with erratic power supply from ZESCO, you decide to play around with galvanic cells and you construct a zinc-copper battery operating at a zero resistant small light emitting diode (L.E.D) at 250C as follows: Zn | Zn (0.10 M) Cu (2.5 M) | Cu The mass of each solid electrode is 2.00 g. Which of the two is a reducing agent? Calculate the cell potential when this battery is first connected. Calculate the cell potential after 10.0 A of current has flowed for 10.0 hours. (Assume cach half-cell contains 1.00 L of solution.) a) b) c)
20. Due to COVID-19 coupled with erratic power supply from ZESCO, you decide to play around with galvanic cells and you construct a zinc-copper battery operating at a zero resistant small light emitting diode (L.E.D) at 250C as follows: Zn | Zn (0.10 M) Cu (2.5 M) | Cu The mass of each solid electrode is 2.00 g. Which of the two is a reducing agent? Calculate the cell potential when this battery is first connected. Calculate the cell potential after 10.0 A of current has flowed for 10.0 hours. (Assume cach half-cell contains 1.00 L of solution.) a) b) c)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Transcribed Image Text:Y Highlight
20. Due to COVID-19 coupled with erratic power supply from ZESCO, you decide to play
around with galvanic cells and you construct a zinc-copper battery operating at a zero
resistant small light emitting diode (L.E.D) at 25°C as follows:
Zn | Zn (0.10 M) Cu (2.5 M) | Cu
The mass of each solid electrode is 2.00 g.
Which of the two is a reducing agent?
Calculate the cell potential when this battery is first connected.
Calculate the cell potential after 10.0 A of current has flowed for 10.0 hours.
(Assume each half-cell contains 1,00L of solution.)
a)
b)
c)
Calculate the mass of each electrode after 10.0 hours.
What will cause the battery to die after some time?
e)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning




Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning