-200 2 Fe + 0, →2 F 2C+02-2 CO -300 -400 -500 -600 -700 4/3 Al + 0, → 2/3 Al,0, -1000 -800 -900 -1100 2 Mg + 0, → 2 MgO -1200 0 400 800 1200 1600 AG° (kJ/mole O2)
-200 2 Fe + 0, →2 F 2C+02-2 CO -300 -400 -500 -600 -700 4/3 Al + 0, → 2/3 Al,0, -1000 -800 -900 -1100 2 Mg + 0, → 2 MgO -1200 0 400 800 1200 1600 AG° (kJ/mole O2)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 128E: In Active Example 3-29 you calculated that you would have to work six weeks to earn enough money to...
Related questions
Question
100%
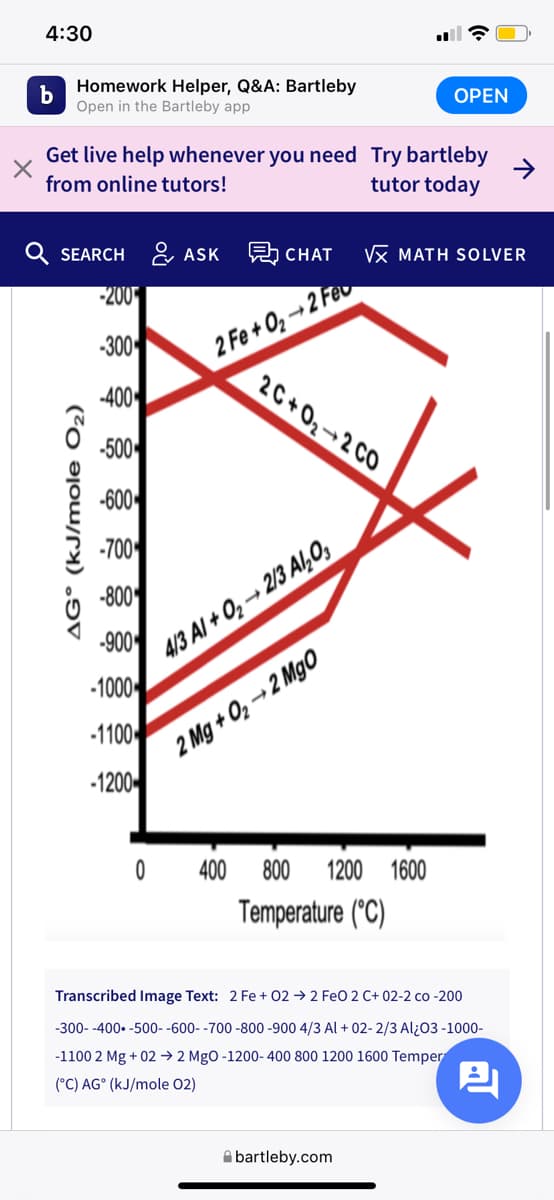
Cite the overall reaction for the extraction of
(a) Fe, (b) Al, and (c) Mg from their corresponding metal oxides when coupled with the oxidation of C (s) to CO (g).
Which of these metals can be extracted from its oxide at 1800 °C?
Transcribed Image Text:4:30
Homework Helper, Q&A: Bartleby
b
Open in the Bartleby app
ОPEN
Get live help whenever you need Try bartleby
from online tutors!
tutor today
Q SEARCH & ASK
A CHAT
Vx MATH SOLVER
2 Fe + 0, →2 Feo
2C+02¬2 CO
-200
-300
-400
-500
-600
-700
4/3 Al + O, → 2/3 Al,0,
-900
-800
-1100
2 Mg + O, → 2 MgO
-1000
-1200
0 400
Temperature (°C)
800
1200 1600
Transcribed Image Text: 2 Fe + 02 → 2 FeO 2 C+ 02-2 co -200
-300- -400. -500- -600- -700 -800 -900 4/3 Al + 02- 2/3 Al¿03 -1000-
-1100 2 Mg + 02 → 2 MgO -1200-400 800 1200 1600 Temper
(°C) AG° (kJ/mole 02)
A bartleby.com
AG° (kJ/m
J/mole O2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
