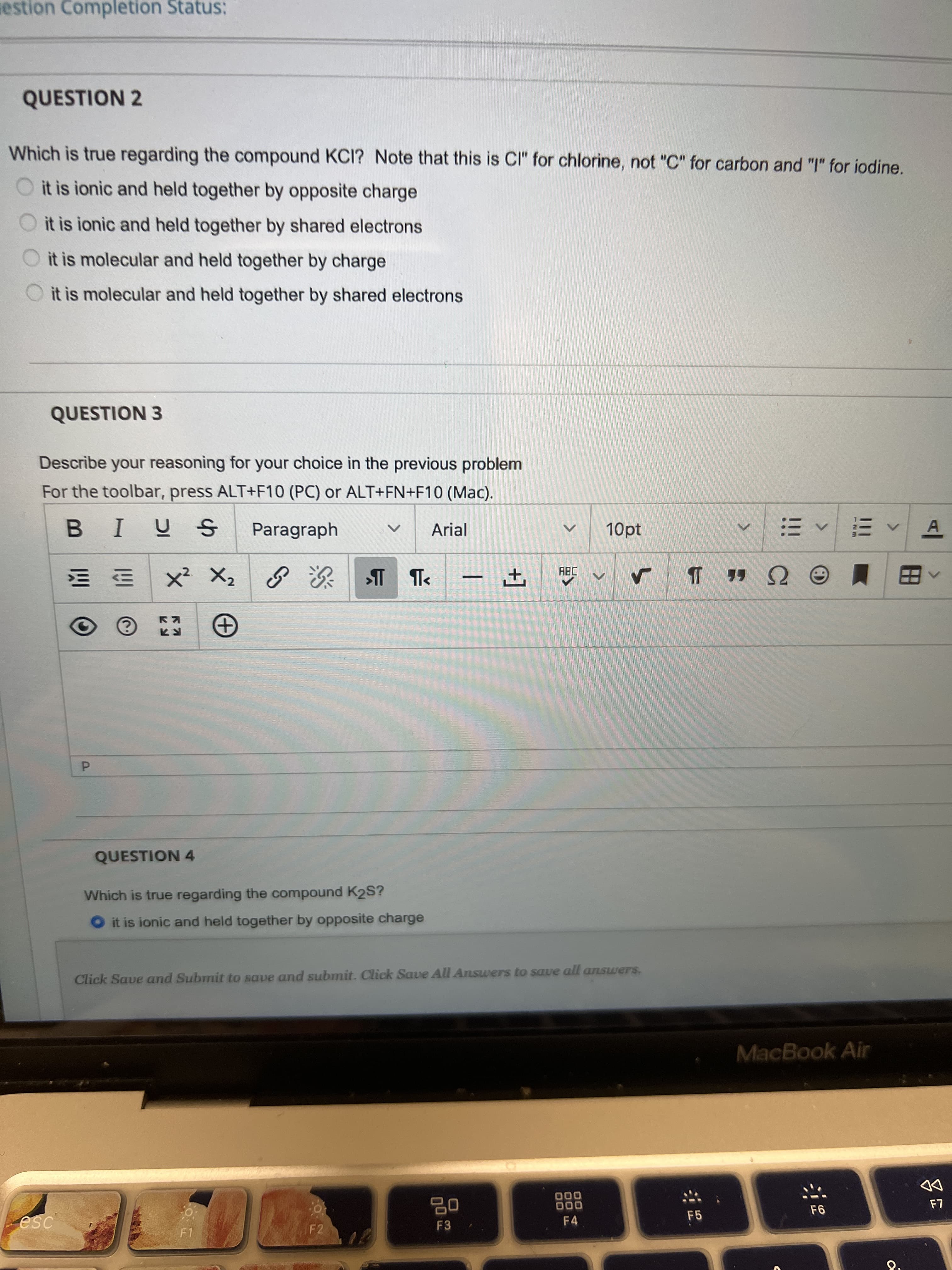
Which is true regarding the compound KCI? Note that this is CI" for chlorine, not "C" for carbon and "I" for iodine. it is ionic and held together by opposite charge O it is ionic and held together by shared electrons O it is molecular and held together by charge it is molecular and held together by shared electrons
Which is true regarding the compound KCI? Note that this is CI" for chlorine, not "C" for carbon and "I" for iodine. it is ionic and held together by opposite charge O it is ionic and held together by shared electrons O it is molecular and held together by charge it is molecular and held together by shared electrons
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter3: Bonding: General Concepts
Section: Chapter Questions
Problem 134AE
Related questions
Question
Transcribed Image Text:II
121
!!!
...
estion Completion Status:
QUESTION 2
Which is true regarding the compound KCI? Note that this is CI" for chlorine, not "C" for carbon and "I" for iodine.
it is ionic and held together by opposite charge
it is ionic and held together by shared electrons
it is molecular and held together by charge
O it is molecular and held together by shared electrons
QUESTION 3
Describe your reasoning for your choice in the previous problem
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
Paragraph
Arial
10pt
x² X, g T Te
ABC
+ –
>田
P.
QUESTION 4
Which is true regarding the compound K2S?
O it is ionic and held together by opposite charge
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
MacBook Air
DD
000
000
F4
F6
F5
esc
F3
F1
F2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning