The Coriolis flowmeter is primarily used to measure the mass flow rate of liquids, although it has also been successfully used in some gas-flow measurement applications. Present the operation principle Coriolis flowmeter.
The Coriolis flowmeter is primarily used to measure the mass flow rate of liquids, although it has also been successfully used in some gas-flow measurement applications. Present the operation principle Coriolis flowmeter.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
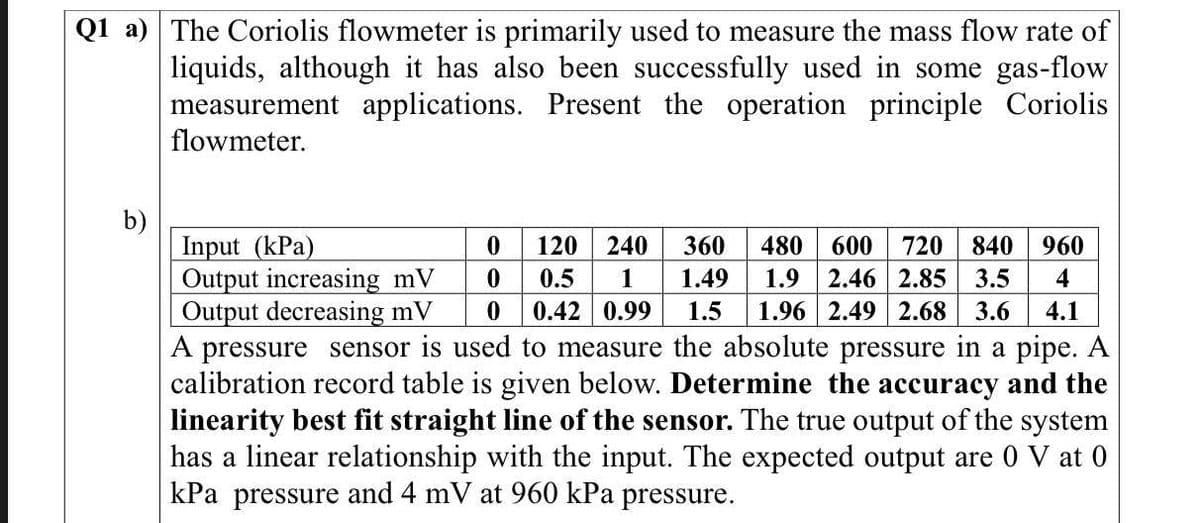
Transcribed Image Text:Q1 a) The Coriolis flowmeter is primarily used to measure the mass flow rate of
liquids, although it has also been successfully used in some gas-flow
measurement applications. Present the operation principle Coriolis
flowmeter.
0
120 240
0.5 1
0 0.42 0.99
0
A pressure sensor is used to measure the absolute pressure in a pipe. A
calibration record table is given below. Determine the accuracy and the
linearity best fit straight line of the sensor. The true output of the system
has a linear relationship with the input. The expected output are 0 V at 0
kPa pressure and 4 mV at 960 kPa pressure.
Input (kPa)
Output increasing mV
Output decreasing mV
360 480 600 720 840 960
1.49 1.9 2.46 2.85 3.5 4
1.5 1.96 2.49 2.68 3.6 4.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The