22 12 50- IR 100- 4000 2 0 11 10 1 3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 3000 integration 9 8 2500 7 2 2 2000 6 5 ppm 1500 -1 Wavenumbers/cm" 3 4 3 2 1000 1 0 500
22 12 50- IR 100- 4000 2 0 11 10 1 3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 3000 integration 9 8 2500 7 2 2 2000 6 5 ppm 1500 -1 Wavenumbers/cm" 3 4 3 2 1000 1 0 500
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter19: Nuclear Magnetic Resonance Spectroscopy
Section: Chapter Questions
Problem 19.8QAP
Related questions
Question
What is the molecular weight, number of carbons,number of hydrogens, molecular formula, and functional group. Also draw the structure.
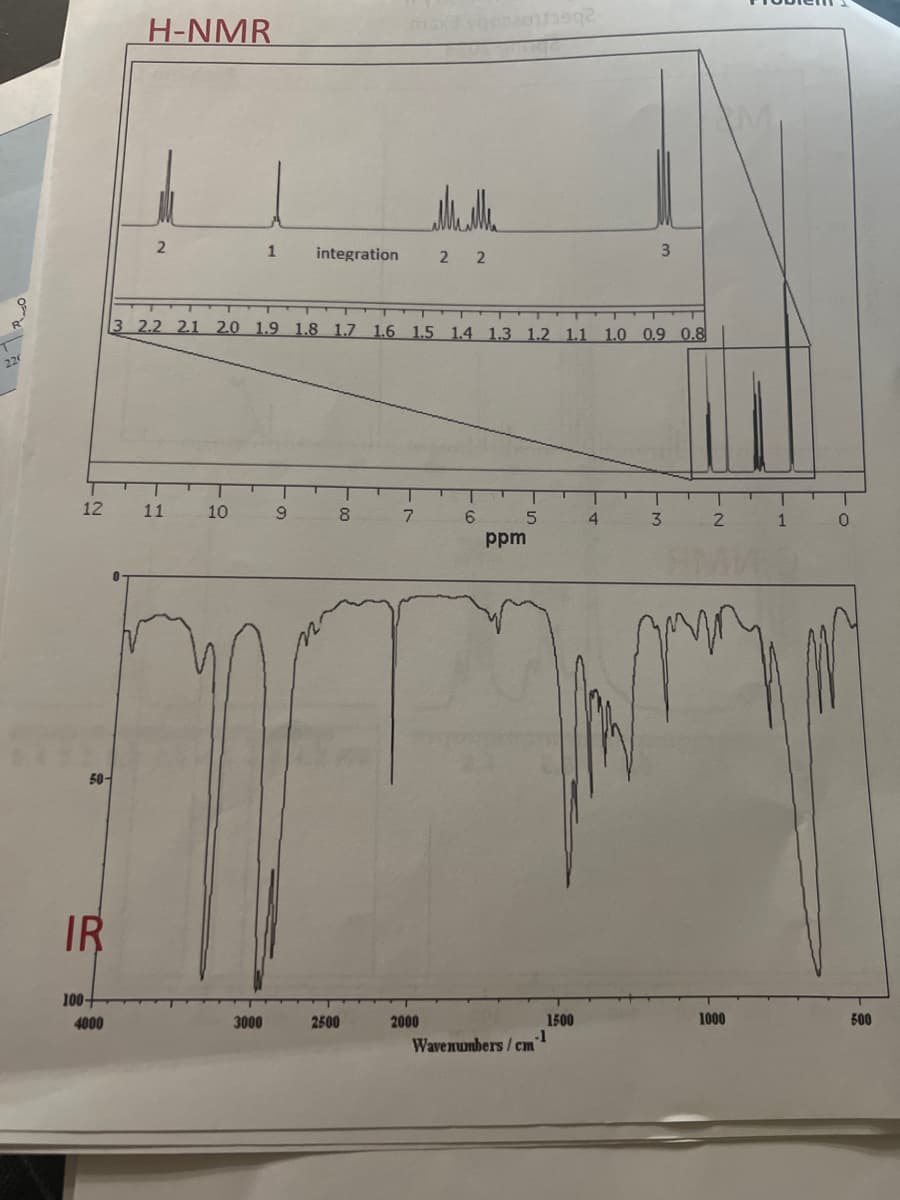
Transcribed Image Text:12
50-
IR
100-
4000
H-NMR
0
2
11
10
1 integration
3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8
3000
9
8
max3 yoobao715902
2500
7
2 2
2000
6
5
ppm
.-1
Wavenumbers/cm
1500
3
4
3
2
1000
T
1
0
500
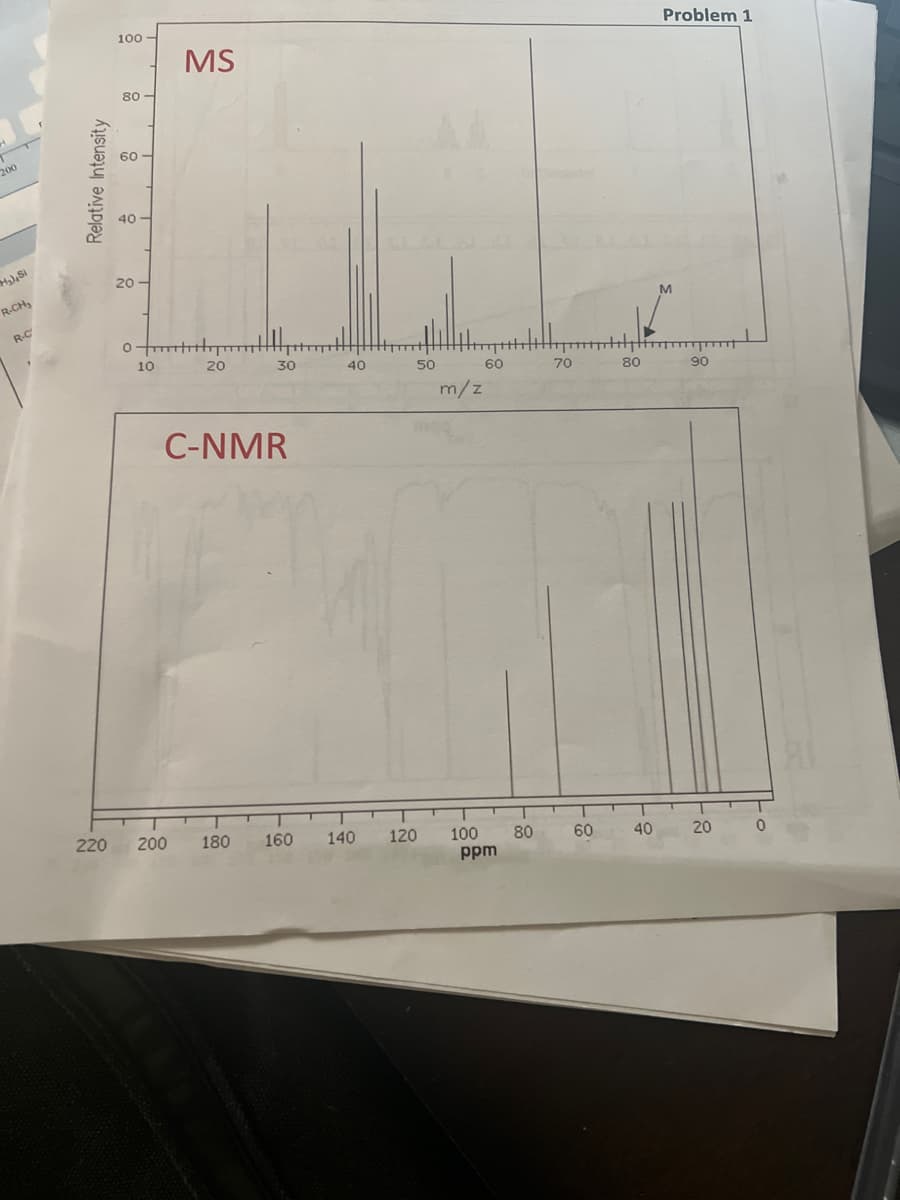
Transcribed Image Text:200
M₂), Si
R-CH₂
R-C
Relative Intensity
220
100
80
60
40-
20
wywo
10
MS
200
20
C-NMR
30
T
180
160
40
140
50
120
m/z
60
100
ppm
80
70
60
80
-
40
Problem 1
M
T
90
20
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

