27.45mLs of 0.250M lead (II) nitrate is reacted with 22.76mLs of 0.450M sodium iodide. How many grams of lead (II) iodide precipitate? (aq) + (aq) → Name Lead (II) nitrate sodium iodide Measurement Conversion moles Calculations (s) + lead (II) iodide not applicable 0.450mol sodium iodide 461.01g 1 L solution 1 mol not applicable not applicable (aq)
27.45mLs of 0.250M lead (II) nitrate is reacted with 22.76mLs of 0.450M sodium iodide. How many grams of lead (II) iodide precipitate? (aq) + (aq) → Name Lead (II) nitrate sodium iodide Measurement Conversion moles Calculations (s) + lead (II) iodide not applicable 0.450mol sodium iodide 461.01g 1 L solution 1 mol not applicable not applicable (aq)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 95AP: 95. Many metal ions form insoluble sulfide compounds when a solution of the metal ion is treated...
Related questions
Question
What is the formula for lead (II) iodide? Surround subscripts with underscores.
Provide steps and explanation
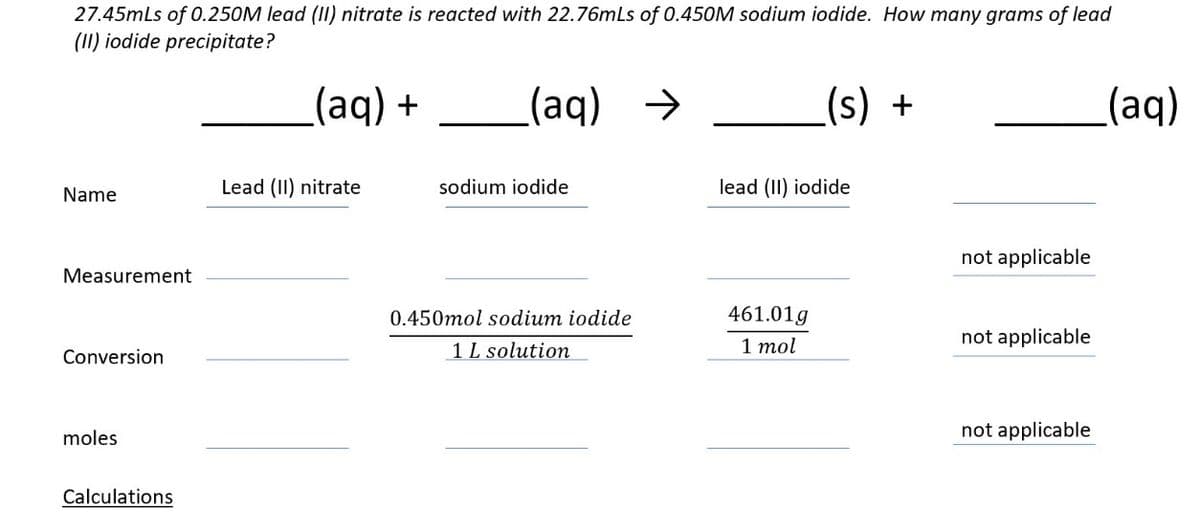
Transcribed Image Text:27.45mLs of 0.250M lead (II) nitrate is reacted with 22.76mLs of 0.450M sodium iodide. How many grams of lead
(II) iodide precipitate?
(aq) +
(aq)
→
(s) +
Name
Lead (II) nitrate
sodium iodide
lead (II) iodide
not applicable
Measurement
0.450mol sodium iodide
461.01g
Conversion
1 L solution
1 mol
not applicable
moles
Calculations
not applicable
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning