29. Which of the following gases cannot be liquefied at 25°C? a. C,H6, critical temperature 562 K b. NH3, critical temperature 405 K c. CO2, critical temperature 304 K d. Cl2, critical temperature 419 K e. O2, critical temperature 155 K 30. The critical temperature and pressure of CO2 are 31°C and 73 atm. Which of the following is true? a. At 31°C, CO, cannot be liquefied no matter how great the pressure b. At 31°C, CO, can be solidified at 150 atm c. At 31°C, CO2 can be liquefied at pressures below 73 atm d. At 31°C, CO2 can be liquefied at pressures greater than 73 atm e. At 31°C, CO2 exists as an equilibrium mixture of solid, liquid and vapor 31. The phase diagram for a pure substance is given below. 300+ 250 Liquid 200 + Pressure, 150 + atm Solid 100+ Vapor 50+ 100 200 300 400 Temperature, K What is the lowest temperature at which liquid can exist? b. 0 K a. 150 K с. 250 K d. 400 K e. 200 K 32. The following unit cell is the b. hexagonally close-packed unit cell d. primitive cubic unit cell a. face-centered cubic unit cell c. body-centered cubic unit cell e. coordination number 8 cubic unit cell
29. Which of the following gases cannot be liquefied at 25°C? a. C,H6, critical temperature 562 K b. NH3, critical temperature 405 K c. CO2, critical temperature 304 K d. Cl2, critical temperature 419 K e. O2, critical temperature 155 K 30. The critical temperature and pressure of CO2 are 31°C and 73 atm. Which of the following is true? a. At 31°C, CO, cannot be liquefied no matter how great the pressure b. At 31°C, CO, can be solidified at 150 atm c. At 31°C, CO2 can be liquefied at pressures below 73 atm d. At 31°C, CO2 can be liquefied at pressures greater than 73 atm e. At 31°C, CO2 exists as an equilibrium mixture of solid, liquid and vapor 31. The phase diagram for a pure substance is given below. 300+ 250 Liquid 200 + Pressure, 150 + atm Solid 100+ Vapor 50+ 100 200 300 400 Temperature, K What is the lowest temperature at which liquid can exist? b. 0 K a. 150 K с. 250 K d. 400 K e. 200 K 32. The following unit cell is the b. hexagonally close-packed unit cell d. primitive cubic unit cell a. face-centered cubic unit cell c. body-centered cubic unit cell e. coordination number 8 cubic unit cell
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section: Chapter Questions
Problem 88GQ: A study of climbers who reached the summit of Mount Everest without supplemental oxygen showed that...
Related questions
Question
Please help me with questions 31 & 32. Thank you in advance
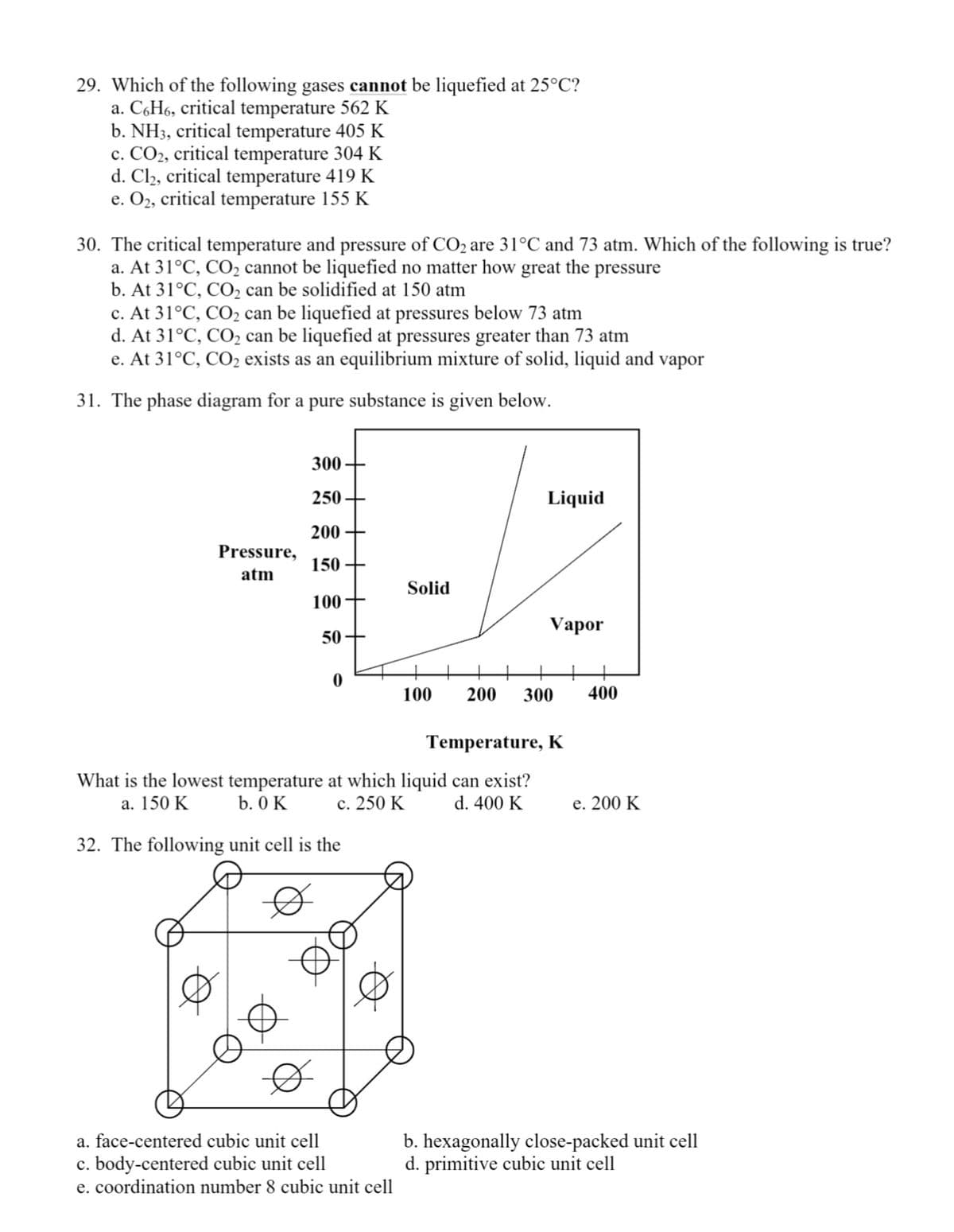
Transcribed Image Text:29. Which of the following gases cannot be liquefied at 25°C?
a. C,H6, critical temperature 562 K
b. NH3, critical temperature 405 K
c. CO2, critical temperature 304 K
d. Cl2, critical temperature 419 K
e. O2, critical temperature 155 K
30. The critical temperature and pressure of CO2 are 31°C and 73 atm. Which of the following is true?
a. At 31°C, CO2 cannot be liquefied no matter how great the pressure
b. At 31°C, CO2 can be solidified at 150 atm
c. At 31°C, CO2 can be liquefied at pressures below 73 atm
d. At 31°C, CO2 can be liquefied at pressures greater than 73 atm
e. At 31°C, CO2 exists as an equilibrium mixture of solid, liquid and vapor
31. The phase diagram for a pure substance is given below.
300
250 +
Liquid
200
Pressure,
150 +
atm
Solid
100
Vapor
50
100
200
300
400
Temperature, K
What is the lowest temperature at which liquid can exist?
b. 0 K
a. 150 K
с. 250 K
d. 400 K
е. 200 K
32. The following unit cell is the
b. hexagonally close-packed unit cell
d. primitive cubic unit cell
a. face-centered cubic unit cell
c. body-centered cubic unit cell
e. coordination number 8 cubic unit cell
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning