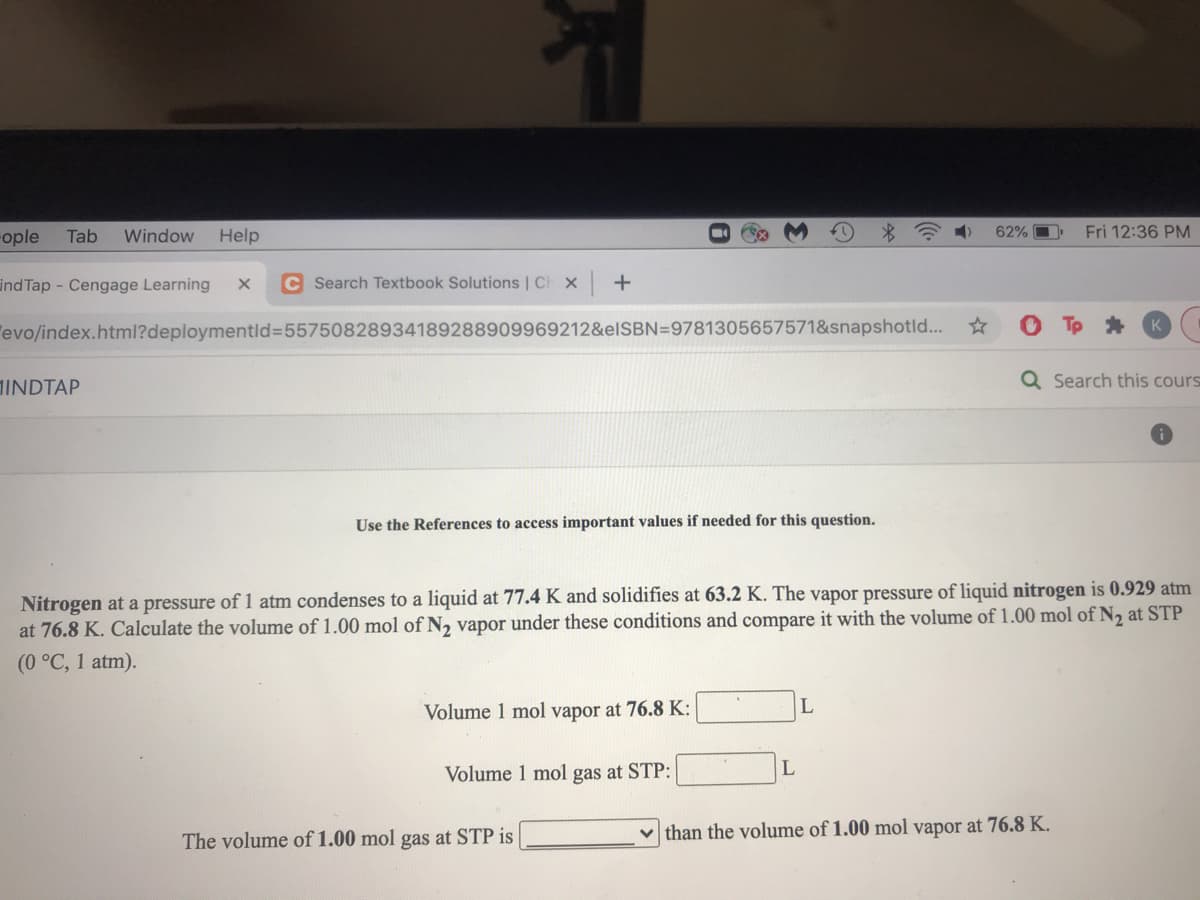
Nitrogen at a pressure of 1 atm condenses to a liquid at 77.4 K and solidifies at 63.2 K. The vapor pressure of liquid nitrogen is 0.929 atm at 76.8 K. Calculate the volume of 1.00 mol of N2 vapor under these conditions and compare it with the volume of 1.00 mol of N2 at STP (0 °C, 1 atm). Volume 1 mol vapor at 76.8 K: Volume 1 mol gas at STP: The volume of 1.00 mol gas at STP is than the volume of 1.00 mol vapor at 76.8 K.
Nitrogen at a pressure of 1 atm condenses to a liquid at 77.4 K and solidifies at 63.2 K. The vapor pressure of liquid nitrogen is 0.929 atm at 76.8 K. Calculate the volume of 1.00 mol of N2 vapor under these conditions and compare it with the volume of 1.00 mol of N2 at STP (0 °C, 1 atm). Volume 1 mol vapor at 76.8 K: Volume 1 mol gas at STP: The volume of 1.00 mol gas at STP is than the volume of 1.00 mol vapor at 76.8 K.
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
Transcribed Image Text:ople
Tab
Window
Help
62% O
Fri 12:36 PM
ind Tap - Cengage Learning
C Search Textbook Solutions | Ch x +
"evo/index.html?deploymentld%3D55750828934189288909969212&elSBN=9781305657571&snapshotld...
INDTAP
Q Search this cours
Use the References to access important values if needed for this question.
Nitrogen at a pressure of 1 atm condenses to a liquid at 77.4 K and solidifies at 63.2 K. The vapor pressure of liquid nitrogen is 0.929 atm
at 76.8 K. Calculate the volume of 1.00 mol of N, vapor under these conditions and compare it with the volume of 1.00 mol of N, at STP
(0 °C, 1 atm).
Volume 1 mol vapor at 76.8 K:
Volume 1 mol gas at STP:
The volume of 1.00 mol gas at STP is
v than the volume of 1.00 mol vapor at 76.8 K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



