2a. EDTA titration of Ca²+ + Mg2+ ions at pH 10 using calmagite indicator Data Trial Trial 2 Volume of tap water sample (accurate to ±0.1 mL) 2.84 mL 2.84 mL mL Volume of 0.01 M EDTA titrant Initial buret reading 0.13 1.05 1.96 mL mL mL Final buret reading 1.05 mL 1.96 mL 2.88 ml Net volume of 0.01 M EDTA titrant 0.92 mL 0.91 mL 0.92 mL Volume of bottled water sample (accurate to ±0.1 mL) 5.57 mL mL mL Volume of 0.01 M EDTA titrant Initial buret reading 3.50 4.12 mL mL mL Final buret reading mL 4.12 4.74 mL Net volume of 0.01 M EDTA titrant 0.62 mL 0.62 mL 0.62 mL Calculate the concentration of Ca²+ + Mg2+ in the tap water and bottled water samples in mmol/L. Show a sam- ple calculation in the space below: C₂· V₂ = 4₁.V₁ Trial 1 C₁.VI 0.01 x 1.05 2.84 = 0.00369 mol → 3.69 mmol/L Calculations Trial Trial 3 Concentration of Ca²+ + Mg2+ in tap water samples mmol/L 10.1 mmol/L Concentration of Ca²+ + Mg²+ in bottled water samples 7.39 8.51 mmol/L mmol/L Tap water average for Ca2+ + Mg2+: mmol/L 7.39 Bottled water average for Ca²+ + Mg²+: mmol/L Using the average value of the Ca²+ + Mg2+ concentrations in millimoles per liter, calculate using Equation (7) the water hardness of the tap water and bottled water in units of mg/L of CaCO3. Show a sample calculation in the space below: mmol My cacos = (Ca²+ + Mg²+) X Imol Cados (mol Ca²+ + My²t) x 100g CaCO3 Mul CaCO3 369 Tap water hardness: ром mg/L CaCO3 (ppm) Bottled water hardness: mg/L CaCO3 (ppm) Tap water- 3.69 mmol/L 3.68 mmol x 369 ppm 0.00369 +0.00690+ 0.0101/3 29-14 1001 5.57 2.88 3.50 3.69 6.28 mol mmol/L mmol/L 6.89 = 5.57 Trial 2 6.90 Trial 3 2.84
2a. EDTA titration of Ca²+ + Mg2+ ions at pH 10 using calmagite indicator Data Trial Trial 2 Volume of tap water sample (accurate to ±0.1 mL) 2.84 mL 2.84 mL mL Volume of 0.01 M EDTA titrant Initial buret reading 0.13 1.05 1.96 mL mL mL Final buret reading 1.05 mL 1.96 mL 2.88 ml Net volume of 0.01 M EDTA titrant 0.92 mL 0.91 mL 0.92 mL Volume of bottled water sample (accurate to ±0.1 mL) 5.57 mL mL mL Volume of 0.01 M EDTA titrant Initial buret reading 3.50 4.12 mL mL mL Final buret reading mL 4.12 4.74 mL Net volume of 0.01 M EDTA titrant 0.62 mL 0.62 mL 0.62 mL Calculate the concentration of Ca²+ + Mg2+ in the tap water and bottled water samples in mmol/L. Show a sam- ple calculation in the space below: C₂· V₂ = 4₁.V₁ Trial 1 C₁.VI 0.01 x 1.05 2.84 = 0.00369 mol → 3.69 mmol/L Calculations Trial Trial 3 Concentration of Ca²+ + Mg2+ in tap water samples mmol/L 10.1 mmol/L Concentration of Ca²+ + Mg²+ in bottled water samples 7.39 8.51 mmol/L mmol/L Tap water average for Ca2+ + Mg2+: mmol/L 7.39 Bottled water average for Ca²+ + Mg²+: mmol/L Using the average value of the Ca²+ + Mg2+ concentrations in millimoles per liter, calculate using Equation (7) the water hardness of the tap water and bottled water in units of mg/L of CaCO3. Show a sample calculation in the space below: mmol My cacos = (Ca²+ + Mg²+) X Imol Cados (mol Ca²+ + My²t) x 100g CaCO3 Mul CaCO3 369 Tap water hardness: ром mg/L CaCO3 (ppm) Bottled water hardness: mg/L CaCO3 (ppm) Tap water- 3.69 mmol/L 3.68 mmol x 369 ppm 0.00369 +0.00690+ 0.0101/3 29-14 1001 5.57 2.88 3.50 3.69 6.28 mol mmol/L mmol/L 6.89 = 5.57 Trial 2 6.90 Trial 3 2.84
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 15.BCP
Related questions
Question
need help with the highlighted portions.
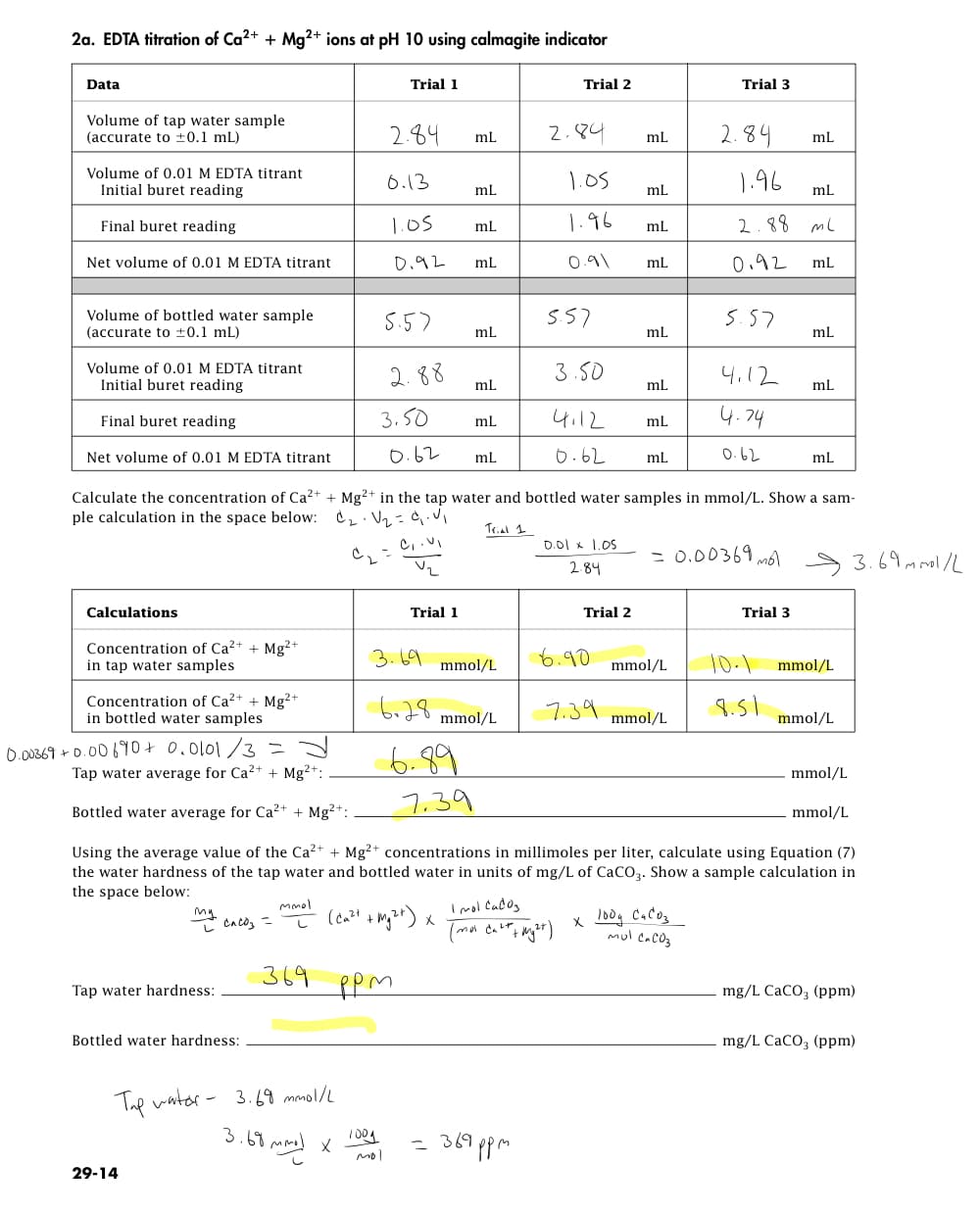
Transcribed Image Text:2a. EDTA titration of Ca²+ + Mg²+ ions at pH 10 using calmagite indicator
Data
Trial
Trial 2
Volume of tap water sample
(accurate to ±0.1 mL)
2.84
mL
2.84
mL
mL
Volume of 0.01 M EDTA titrant
Initial buret reading
0.13
1.05
1.96
mL
mL
mL
Final buret reading
1.05
mL
1.96
mL
2.88 ml
Net volume of 0.01 M EDTA titrant
0.92
mL
0.91
mL
0.92
mL
Volume of bottled water sample
(accurate to ±0.1 mL)
5.57
mL
mL
mL
Volume of 0.01 M EDTA titrant
Initial buret reading
3.50
4.12
mL
mL
mL
Final buret reading
3.50
mL
4.12
4.74
mL
Net volume of 0.01 M EDTA titrant
0.62 mL
0.62
mL
0.62
mL
Calculate the concentration of Ca²+ + Mg2+ in the tap water and bottled water samples in mmol/L. Show a sam-
ple calculation in the space below: C₂·V₂ = 4₁.V₁
Trial 1
C₁.VI
0.01 x 1.05
2.84
= 0.00369 mol 3.69 mmol/L
Calculations
Trial
Trial 3
Concentration of Ca²+ + Mg2+
in tap water samples
mmol/L
10-1 mmol/L
Concentration of Ca²+ + Mg²+
in bottled water samples
7.39
8.51
mmol/L
mmol/L
Tap water average for Ca2+ + Mg²+:
mmol/L
7.39
Bottled water average for Ca²+ + Mg²+:
mmol/L
Using the average value of the Ca²+ + Mg2+ concentrations in millimoles per liter, calculate using Equation (7)
the water hardness of the tap water and bottled water in units of mg/L of CaCO3. Show a sample calculation in
the space below:
mmol
my cnco₂ =
(Ca²+ + Mg²+) X
Imol Cadog
(mol Cart + My²t)
x 100g CaCO3
Mul CaCO3
Tap water hardness:
ром
mg/L CaCO3 (ppm)
Bottled water hardness:
mg/L CaCO3 (ppm)
Tap water-
369 ppm
0.00369 +0.00690+ 0.0101/3
29-14
369
3.69 mmol/L
3.68 mmol x
3.69
1001
5.57
2.88
6.28
mol
mmol/L
mmol/L
6.89
=
5.57
Trial 2
6.90
Trial 3
2.84
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

