2furoic adid isa white solid that follows this usual pattern as, 1 gram of 24uroic acid disolves in • 26 m of water at room temperature 4mi of (100 C boling water The amount of water needed to recrystallize 4.13g furoic acid at 100 'Cis ml. Upon cooling the atbove solution to the room temperature, the amount of furoic acid that will stay dissoved in the mother liquer is The amount of furaic acid that will recrystalue at room temperature from the above experiment is The manimum theoretical recovery from this process is
2furoic adid isa white solid that follows this usual pattern as, 1 gram of 24uroic acid disolves in • 26 m of water at room temperature 4mi of (100 C boling water The amount of water needed to recrystallize 4.13g furoic acid at 100 'Cis ml. Upon cooling the atbove solution to the room temperature, the amount of furoic acid that will stay dissoved in the mother liquer is The amount of furaic acid that will recrystalue at room temperature from the above experiment is The manimum theoretical recovery from this process is
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 103QRT
Related questions
Question
6
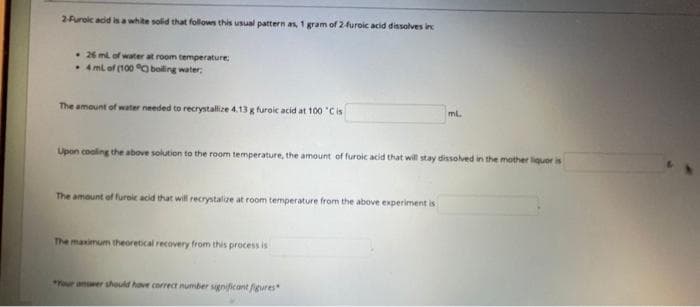
Transcribed Image Text:2Furoic acid
white solid that folows this usual pattern as, 1 gram of 2furoic acid dissolves inc
• 26 m of water at room temperature
• 4 ml of (100 O boling water,
The amount of water needed to recrystallize 4.13 g furoic acid at 100 'Cis
ml
Upon cooling the above solution to the room temperature, the amount of furoic acid that will stay dissolved in the mother liquor is
The amount of furoic acid that will recrystalize at room temperature from the above experiment is
The maximum theoretical recovery from this process is
*Your answer should have correct number gnificant fgures
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole