2HNO3(aq) → Pb(NO3)2(aq) + H2O(1) + CO2(g) q) + 2HC1(aq) → 2HNO3(aq) + PbCl2(s) dent starts with 2.871 g of lead(II) carbonate for the first reaction and all nts are added in excess, what is the theoretical yield of lead(II) chloride
2HNO3(aq) → Pb(NO3)2(aq) + H2O(1) + CO2(g) q) + 2HC1(aq) → 2HNO3(aq) + PbCl2(s) dent starts with 2.871 g of lead(II) carbonate for the first reaction and all nts are added in excess, what is the theoretical yield of lead(II) chloride
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 90GQ: A Menthol, from oil of mint, has a characteristic odor. The compound contains only C, H, and O. If...
Related questions
Question
How do I solve this
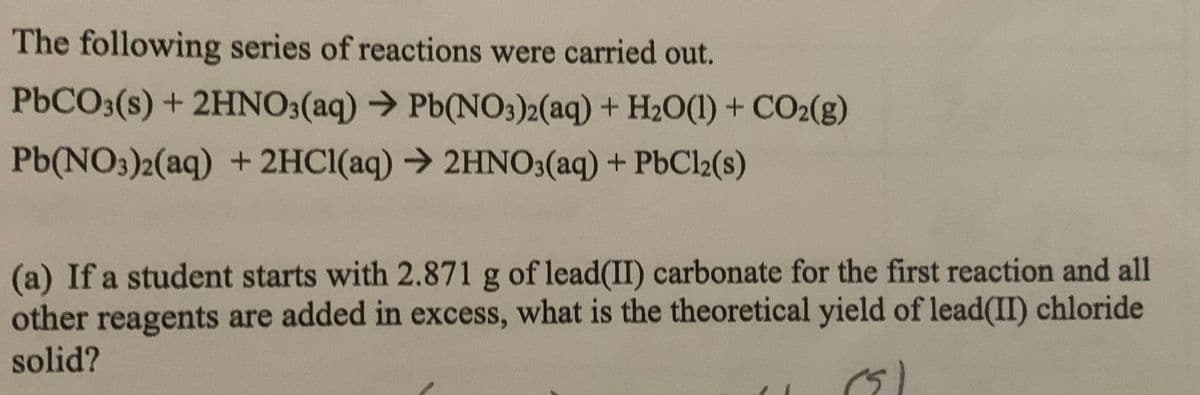
Transcribed Image Text:The following series of reactions were carried out.
P6CO3(s) + 2HNO3(aq) → Pb(NO3)2(aq) + H2O(1) + CO2(g)
Pb(NO3)2(aq) + 2HCI(aq) → 2HNO3(aq) + PbCl2(s)
(a) If a student starts with 2.871 g of lead(II) carbonate for the first reaction and all
other reagents are added in excess, what is the theoretical yield of lead(II) chloride
solid?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning