3- In its ground state, the hydrogen atom can be modeled as a positive point charge of magnitude +e (the proton) surrounded by a negative charge distribution with a charge density that varies with the distance from the center of the proton r as p = Poe¯2r/a, where a = 0.523nm is the most likely distance from the electron to the proton. (a) Calculate po necessary for the hydrogen atom to be neutral. (b) Calculate the electrostatic potential (relative to infinity) of this system as a function of the distance r to the proton.
3- In its ground state, the hydrogen atom can be modeled as a positive point charge of magnitude +e (the proton) surrounded by a negative charge distribution with a charge density that varies with the distance from the center of the proton r as p = Poe¯2r/a, where a = 0.523nm is the most likely distance from the electron to the proton. (a) Calculate po necessary for the hydrogen atom to be neutral. (b) Calculate the electrostatic potential (relative to infinity) of this system as a function of the distance r to the proton.
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter9: Atomic Structure
Section: Chapter Questions
Problem 7P
Related questions
Question
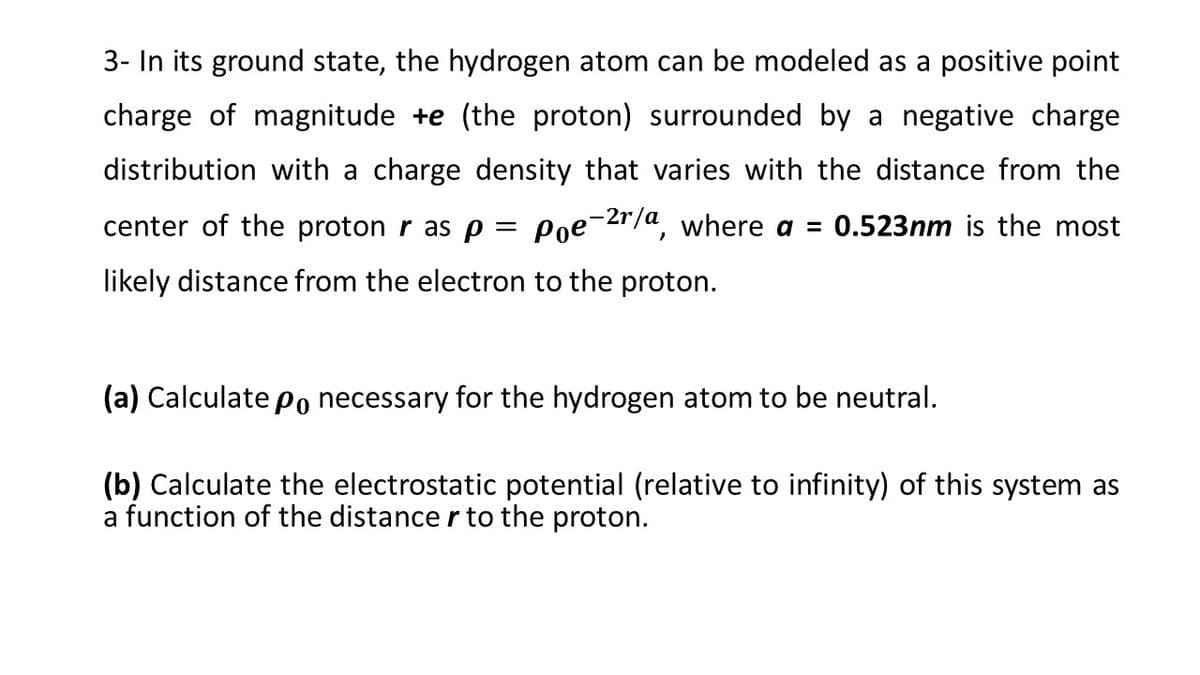
Transcribed Image Text:3- In its ground state, the hydrogen atom can be modeled as a positive point
charge of magnitude +e (the proton) surrounded by a negative charge
distribution with a charge density that varies with the distance from the
center of the proton r as p = Poe-2r/a, where a = 0.523nm is the most
likely distance from the electron to the proton.
(a) Calculate Po necessary for the hydrogen atom to be neutral.
(b) Calculate the electrostatic potential (relative to infinity) of this system as
a function of the distance r to the proton.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University