An electron in the n = 1 energy orbital of the hydrogen atom has an angular momentum of magnitude 1.055 x 10-34 J-s and an orbital radius of 5.292 x 10-1l m. The electron's mass is 9.11 x 10-31 kg. What is the electron's speed v in this orbital? U = m/s
An electron in the n = 1 energy orbital of the hydrogen atom has an angular momentum of magnitude 1.055 x 10-34 J-s and an orbital radius of 5.292 x 10-1l m. The electron's mass is 9.11 x 10-31 kg. What is the electron's speed v in this orbital? U = m/s
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter39: Relativity
Section: Chapter Questions
Problem 39.1OQ: (i) Does the speed of an electron have an upper limit? (a) yes, the speed of light c (b) yes, with...
Related questions
Question
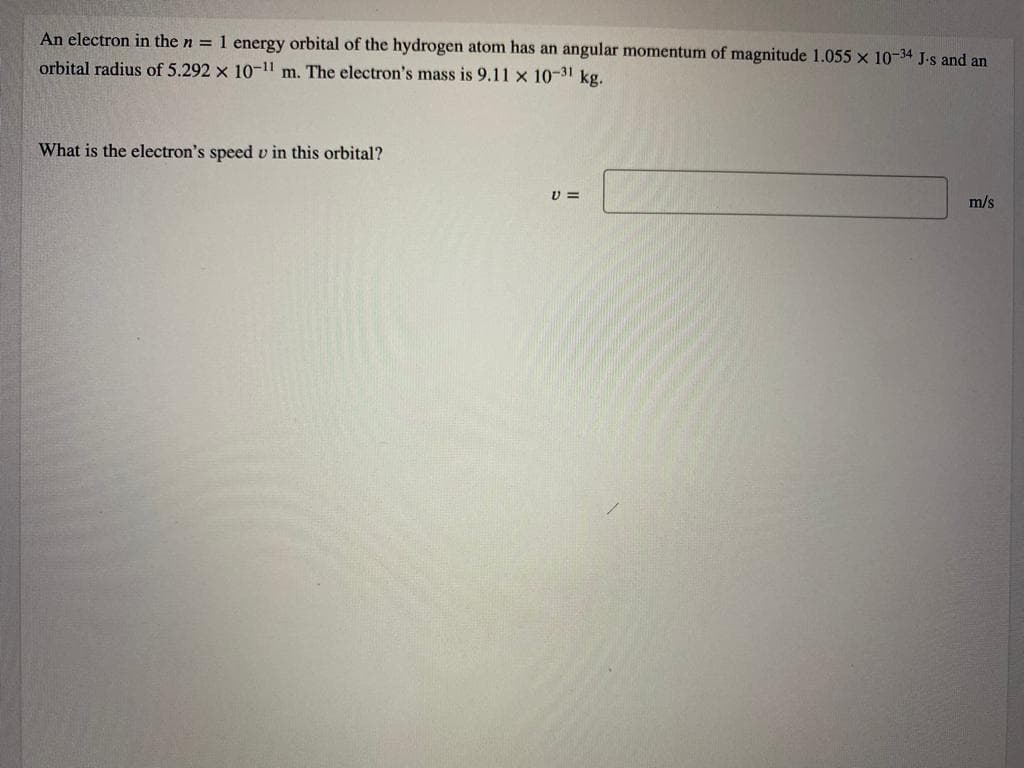
Transcribed Image Text:An electron in the n = 1 energy orbital of the hydrogen atom has an angular momentum of magnitude 1.055 x 10-34 J.s and an
orbital radius of 5.292 x 10-11 m. The electron's mass is 9.11 x 10-31 kg.
What is the electron's speed v in this orbital?
m/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning