3-Practically, the third equivalence point of H3PO4 will not appear in the titration curve. O The jump is very small to be detected on pH meter. The conjugate base (PO4 3-) is a strong base that is able to give HPO4 2- + OH- after puling an H+ from H20 O H3P04 is able to dissociate only for two times O The conjugate base (PO4 3-) is a weak base and therefore undetectable
3-Practically, the third equivalence point of H3PO4 will not appear in the titration curve. O The jump is very small to be detected on pH meter. The conjugate base (PO4 3-) is a strong base that is able to give HPO4 2- + OH- after puling an H+ from H20 O H3P04 is able to dissociate only for two times O The conjugate base (PO4 3-) is a weak base and therefore undetectable
Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.29QAP
Related questions
Question
Transcribed Image Text:C Acids
O d) 8
3-Practically, the third equivalence point of H3PO4 will not appear in the titration
curve.
O The jump is very small to be detected on pH meter.
The conjugate base (PO4 3-) is a strong base that is able to give HPO4 2- + OH-
after puling an H+ from H20
H3P04 is able to dissociate only for two times
O The conjugate base (PO4 3-) is a weak base and therefore undetectable
Submit
Page 1 of
University Renort Abuse
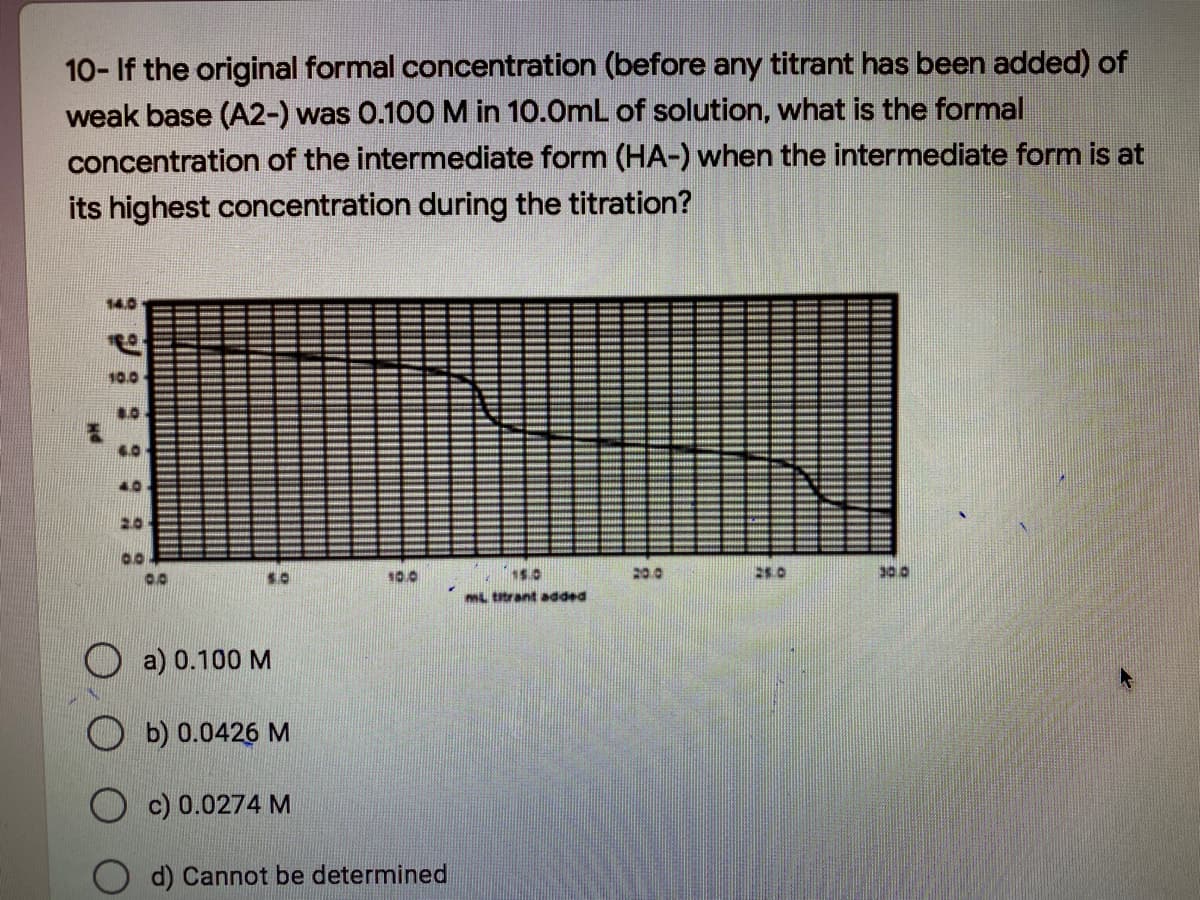
Transcribed Image Text:10- If the original formal concentration (before any titrant has been added) of
weak base (A2-) was 0.100M in 10.0mL of solution, what is the formal
concentration of the intermediate form (HA-) when the intermediate form is at
its highest concentration during the titration?
14.0
- --
---
10.0
.0
---
--- -
-- --
- ---
4.0
- --
- - -- --
---i
-- *
4.0
- --
---
- --- ---
50
10.0
20.0
ESO
300
mL titrant added
O a) 0.100 M
O b) 0.0426 M
O c) 0.0274 M
d) Cannot be determined
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning