A 0.2521-g sample of an unknown weak acid is titrated with a 0.1005 M NaOH, requiring 42.68 mL to reach the phenolphthalein end point. Determine the compound's equivalent weight. Which of the following compounds is most likely to be the unknown weak acid? Name Formula MM, g/mol Туре Ascorbic acid C6H8O6 176.1 Monoprotic Malonic acid C3H4O4 104.1 Diprotic Succinic acid C,HO4 118.1 Diprotic Citric acid C6H§O7 192.1 Triprotic
A 0.2521-g sample of an unknown weak acid is titrated with a 0.1005 M NaOH, requiring 42.68 mL to reach the phenolphthalein end point. Determine the compound's equivalent weight. Which of the following compounds is most likely to be the unknown weak acid? Name Formula MM, g/mol Туре Ascorbic acid C6H8O6 176.1 Monoprotic Malonic acid C3H4O4 104.1 Diprotic Succinic acid C,HO4 118.1 Diprotic Citric acid C6H§O7 192.1 Triprotic
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 74QAP: Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a)...
Related questions
Question
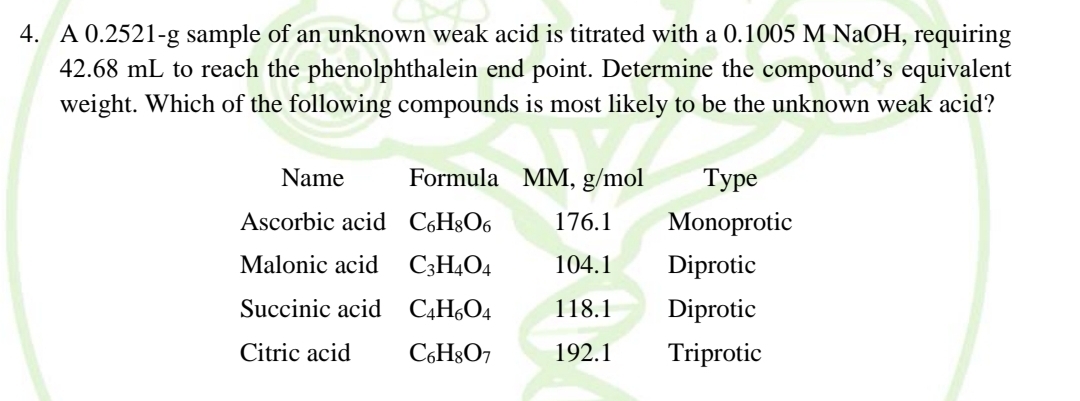
Transcribed Image Text:4. A 0.2521-g sample of an unknown weak acid is titrated with a 0.1005 M NaOH, requiring
42.68 mL to reach the phenolphthalein end point. Determine the compound's equivalent
weight. Which of the following compounds is most likely to be the unknown weak acid?
Name
Formula MM, g/mol
Туре
Ascorbic acid C6H8O6
176.1
Monoprotic
Malonic acid C;H4O4
104.1
Diprotic
Succinic acid C,H¿O4
118.1
Diprotic
Citric acid
C,H8O7
192.1
Triprotic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning