Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.15QAP
Related questions
Question
How can I solve the rest of these equations for question 3?
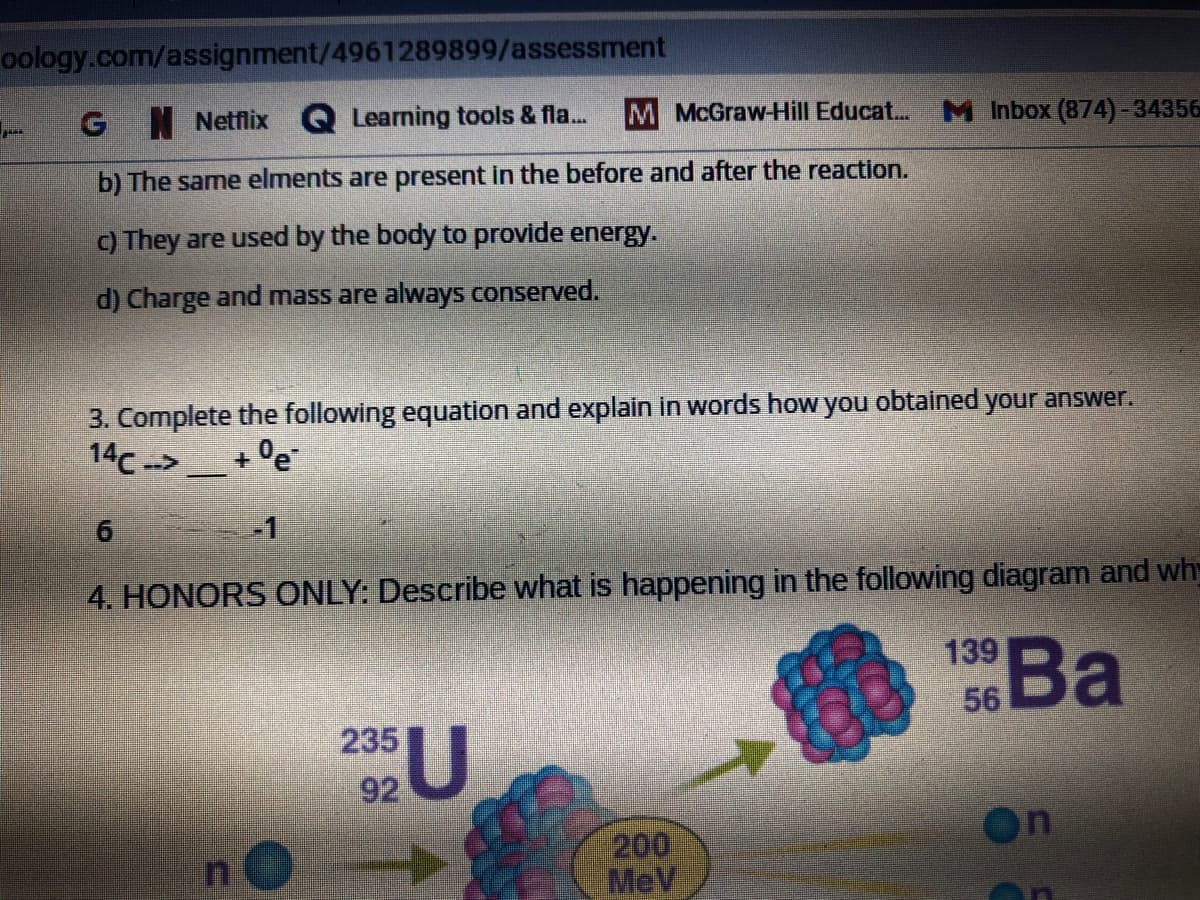
Transcribed Image Text:oology.com/assignment/4961289899/assessment
G N Netflix
Learning tools & fla..
M McGraw-Hill Educat...
M Inbox (874)-34356
b) The same elments are present in the before and after the reaction.
C) They are used by the body to provide energy.
d) Charge and mass are always conserved.
3. Complete the following equation and explain in words how you obtained your answer.
14c ->
+ °e
4. HONORS ONLY: Describe what is happening in the following diagram and wh
Ва
139
se Ba
56
235
92U
200
MeV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning