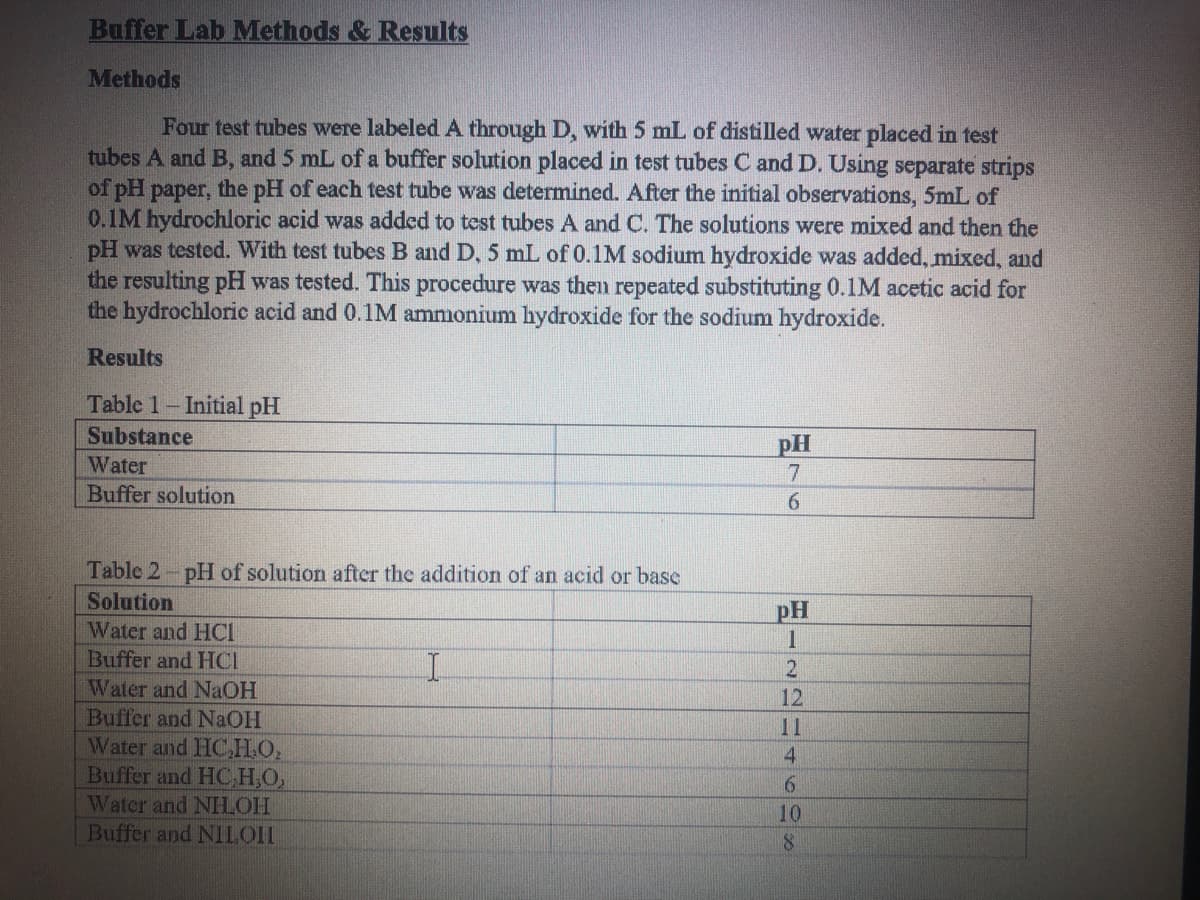
Buffer Lab Methods & Results Methods Four test tubes were labeled A through D, with 5 mL of distilled water placed in test tubes A and B, and 5 mL of a buffer solution placed in test tubes C and D. Using separate strips of pH paper, the pH of each test tube was determined. After the initial observations, 5mL of 0.1M hydrochloric acid was added to test tubes A and C. The solutions were mixed and then the pH was tested. With test tubes B and D, 5 mL of 0.1M sodium hydroxide was added, mixed, and the resulting pH was tested. This procedure was then repeated substituting 0.1M acetic acid for the hydrochloric acid and 0.1M ammonium hydroxide for the sodium hydroxide. Results Table 1- Initial pH Substance pH Water 7 Buffer solution 6. Table 2-pH of solution after the addition of an acid or base Solution pH Water and HCI Buffer and HCI Water and NaOH Buffer and NaOH Water and HC,H.O. Buffer and HOНО. Watcr and NHLOH Buffer and NILOI 12 11 4. 6. 10
Atmospheric Pollution
In the atmosphere, the existence of large quantities of undesirable substances that could cause several health issues to living organisms and humans is atmospheric pollution. Air pollution is otherwise known to be atmospheric pollution. The presence of undesirable materials would also destruct the natural environment such as a change in climate, degradation of habitat, or depletion of ozone. Air pollution is generated by the natural processes and activities of humans.
Smokestack Scrubbers
Once we believe in the environment and remember the pollution-producing facets of it, we will consider the smoke-stacks scrubber to be a number of the worst offenders. Although this is often legally correct, smoke-stack scrubbers often have a big function in terms of keeping ground-level air pollutants- safe to breathe and assisting within the management of emissions.
Step by step
Solved in 3 steps









