3. Consider the structure of N,N-dimethylformamide (DMF, seen below). We might expect the two methyl groups to be equivalent; however, both the proton and carbon NMR spectra of DMF show two separate signals for the methyl groups. Propose an explanation for the nonequivalence of the methyl groups. (HINT-Consider the resonance contributors of this compound) Should be CH3 Hi CH3
3. Consider the structure of N,N-dimethylformamide (DMF, seen below). We might expect the two methyl groups to be equivalent; however, both the proton and carbon NMR spectra of DMF show two separate signals for the methyl groups. Propose an explanation for the nonequivalence of the methyl groups. (HINT-Consider the resonance contributors of this compound) Should be CH3 Hi CH3
Chapter13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy
Section13.SE: Something Extra
Problem 56GP: Long-range coupling between protons more than two carbon atoms apart is sometimes observed when ...
Related questions
Question
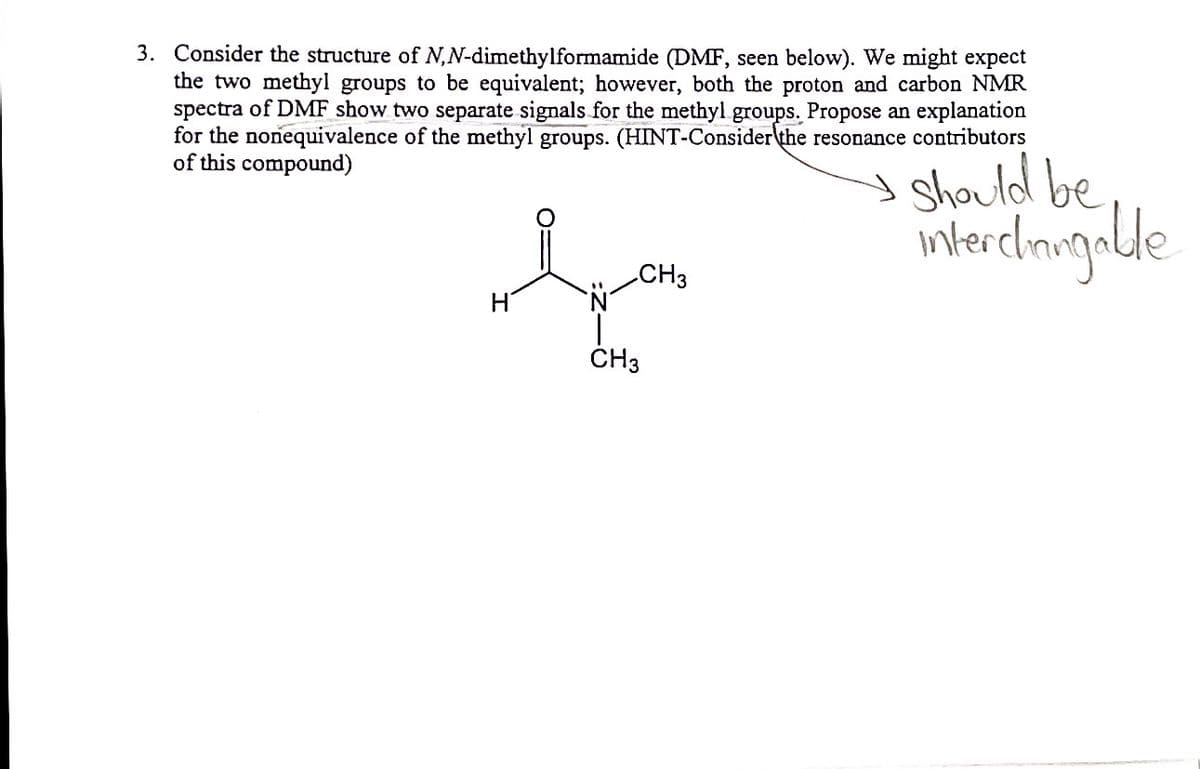
Transcribed Image Text:3. Consider the structure of N,N-dimethylformamide (DMF, seen below). We might expect
the two methyl groups to be equivalent; however, both the proton and carbon NMR
spectra of DMF show two separate signals for the methyl groups. Propose an explanation
for the nonequivalence of the methyl groups. (HINT-Consider\the resonance contributors
of this compound)
I Should be
intercimigalde
CH3
ČH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole