3. For the Carnot engine cycle for a system containing one mole of ideal gas (p,V data points given below), calculate the change in entropy of the system for each stage of the cycle (Steps 1, 2, 3, and 4) and the overall change in entropy of the system for a complete cycle. You may assume that each measurement of the data is accurate to at least two significant figures. Data Points (p,V): Point A: p = 70 kPa, V = 0.100 m3 Point B: p = 35 kPa, V = 0.200 m3 Point C: p = 10 kPa, V = 0.400 m³ Point D: p = 20 kPa, V = 0.200 m3 Step 1: Isothermal expansion from point A to point B. Step 2: Adiabatic expansion from point B to point C. Step 3: Isothermal compression from point C to point D. Step 4: Adiabatic compression from point D back to point A.
3. For the Carnot engine cycle for a system containing one mole of ideal gas (p,V data points given below), calculate the change in entropy of the system for each stage of the cycle (Steps 1, 2, 3, and 4) and the overall change in entropy of the system for a complete cycle. You may assume that each measurement of the data is accurate to at least two significant figures. Data Points (p,V): Point A: p = 70 kPa, V = 0.100 m3 Point B: p = 35 kPa, V = 0.200 m3 Point C: p = 10 kPa, V = 0.400 m³ Point D: p = 20 kPa, V = 0.200 m3 Step 1: Isothermal expansion from point A to point B. Step 2: Adiabatic expansion from point B to point C. Step 3: Isothermal compression from point C to point D. Step 4: Adiabatic compression from point D back to point A.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
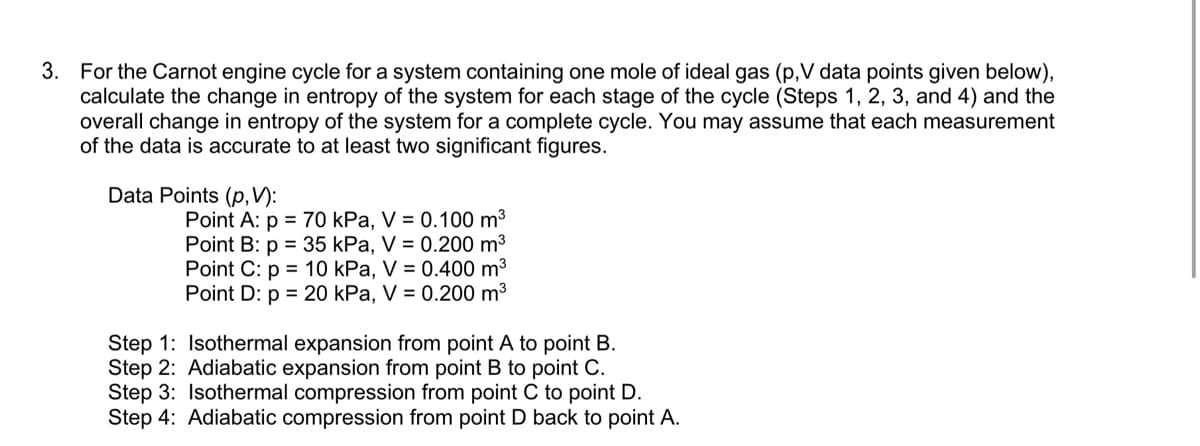
Transcribed Image Text:3. For the Carnot engine cycle for a system containing one mole of ideal gas (p,V data points given below),
calculate the change in entropy of the system for each stage of the cycle (Steps 1, 2, 3, and 4) and the
overall change in entropy of the system for a complete cycle. You may assume that each measurement
of the data is accurate to at least two significant figures.
Data Points (p,V):
Point A: p = 70 kPa, V = 0.100 m3
Point B: p = 35 kPa, V = 0.200 m3
Point C: p = 10 kPa, V = 0.400 m3
Point D: p = 20 kPa, V = 0.200 m3
Step 1: Isothermal expansion from point A to point B.
Step 2: Adiabatic expansion from point B to point C.
Step 3: Isothermal compression from point C to point D.
Step 4: Adiabatic compression from point D back to point A.
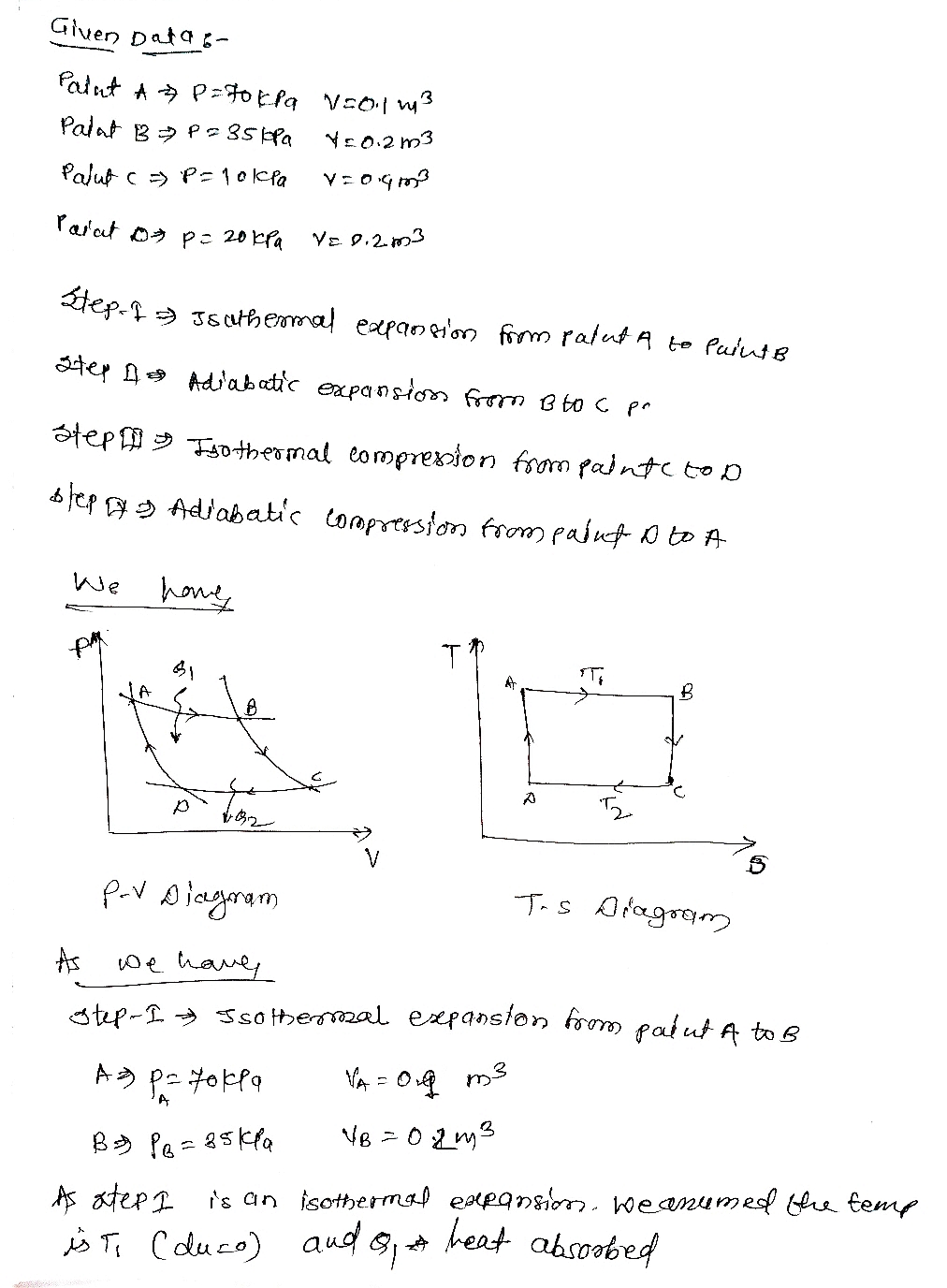
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY