3. How many grams and atoms of carbon are present in 500 grams of acetaminophen (C₂H9NO₂), which has a molar mass relative to your answer from number 2? Show your solution in dimensional analysis below. (1 mole = 6.022 x 10²3 particles) Mole of C atoms= Mass of Catoms= Number of C atoms= mol .8 particles 4. An unknown compound is analyzed and found to consist of 24.3 % carbon, 4.1 % hydrogen, and 71.6 % chlorine. If the molar mass of the compound is 98.8 g/mol, what is the molecular formula of the compound?
3. How many grams and atoms of carbon are present in 500 grams of acetaminophen (C₂H9NO₂), which has a molar mass relative to your answer from number 2? Show your solution in dimensional analysis below. (1 mole = 6.022 x 10²3 particles) Mole of C atoms= Mass of Catoms= Number of C atoms= mol .8 particles 4. An unknown compound is analyzed and found to consist of 24.3 % carbon, 4.1 % hydrogen, and 71.6 % chlorine. If the molar mass of the compound is 98.8 g/mol, what is the molecular formula of the compound?
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
PLEASE ANSWER 3 and 4 ONLY PLEASE ASAP
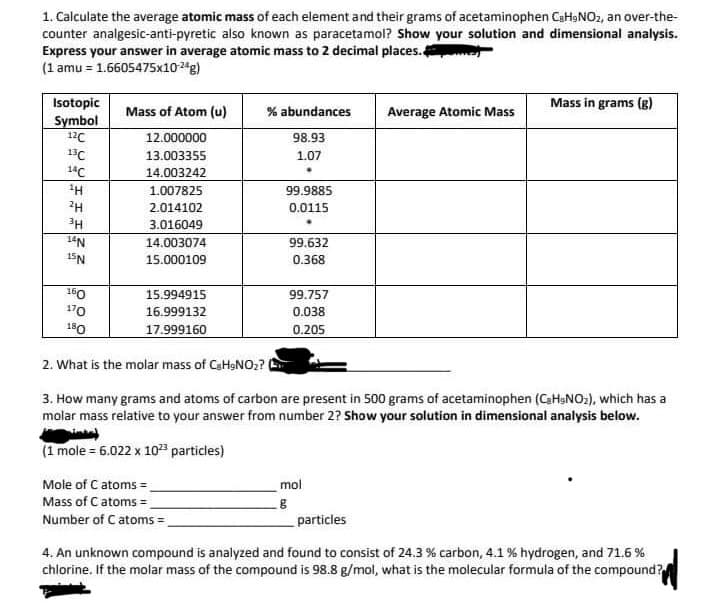
Transcribed Image Text:1. Calculate the average atomic mass of each element and their grams of acetaminophen C₂H₂NO₂, an over-the-
counter analgesic-anti-pyretic also known as paracetamol? Show your solution and dimensional analysis.
Express your answer in average atomic mass to 2 decimal places.
(1 amu = 1.6605475x10-24g)
Isotopic
Symbol
12C
13C
14C
¹H
2H
3H
14N
15N
160
170
180
Mass of Atom (u)
12.000000
13.003355
14.003242
1.007825
2.014102
3.016049
14.003074
15.000109
15.994915
16.999132
17.999160
% abundances
98.93
1.07
Mole of C atoms=_
Mass of C atoms =
Number of C atoms=_
99.9885
0.0115
99.632
0.368
99.757
0.038
0.205
Average Atomic Mass
2. What is the molar mass of CaHaNO₂? (
3. How many grams and atoms of carbon are present in 500 grams of acetaminophen (C₂H₂NO₂), which has a
molar mass relative to your answer from number 2? Show your solution in dimensional analysis below.
(1 mole = 6.022 x 10²³ particles)
mol
g
Mass in grams (g)
particles
4. An unknown compound is analyzed and found to consist of 24.3 % carbon, 4.1 % hydrogen, and 71.6 %
chlorine. If the molar mass of the compound is 98.8 g/mol, what is the molecular formula of the compound?
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

