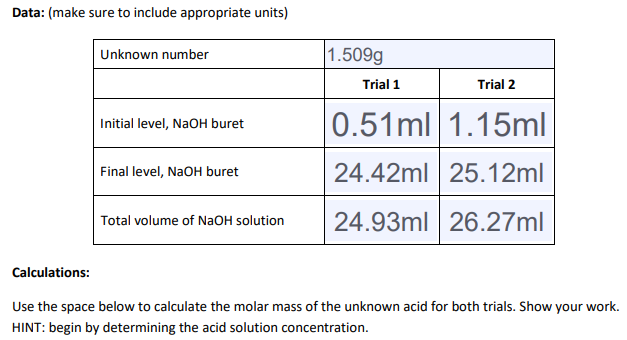
Data: (make sure to include appropriate units) Unknown number 1.509g Trial 1 Trial 2 0.51ml 1.15ml Initial level, NaOH buret Final level, NaOH buret 24.42ml 25.12ml 24.93ml 26.27ml Total volume of NAOH solution Calculations: Use the space below to calculate the molar mass of the unknown acid for both trials. Show your work. HINT: begin by determining the acid solution concentration.
A student weighed approximately 1.5 g of an unknown solid acid and carefully placed the sample in a clean, but not necessarily dry, 250.0 mL volumetric flask. The student added deionized water to the flask, swirled the solution until the solid acid dissolved, then added sufficient deionized water to fill the flask to the fill line. A volumetric pipet was used to transfer 25.00 mL of the resulting solution to an Erlenmeyer flask, and 2 – 3 drops of phenolphthalein were added.
The student filled the sodium hydroxide buret and titrated the acid solution with standardized 0.1000 M NaOH to the first permanent faint pink color, then repeated the titration a second time.
Your instructor will provide the mass of unknown solid acid, as well as the initial and final buret readings for the two titrations. From this data and the information above, you will be able to calculate the molar mass of the unknown acid.
The unknown solid acid is diprotic; that is, there are two moles of ionizable H+ ions per mole of solid acid.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images


