3. In both the ammonia, NH3, synthesis and the production of SO3, according to ... N2(g) + 3 H2(g) 2 NH3(g) AH = - 92 kJ 2 SO2(g) + O2(g) 2 SO3(g) AH = - 180 kJ The mole fraction at equilibrium of the desired product (NH3, and SO3), is greater at lower temperatures. Yet, in the commercial production of these substances relatively high temperatures are used. Explain why this is so.
3. In both the ammonia, NH3, synthesis and the production of SO3, according to ... N2(g) + 3 H2(g) 2 NH3(g) AH = - 92 kJ 2 SO2(g) + O2(g) 2 SO3(g) AH = - 180 kJ The mole fraction at equilibrium of the desired product (NH3, and SO3), is greater at lower temperatures. Yet, in the commercial production of these substances relatively high temperatures are used. Explain why this is so.
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
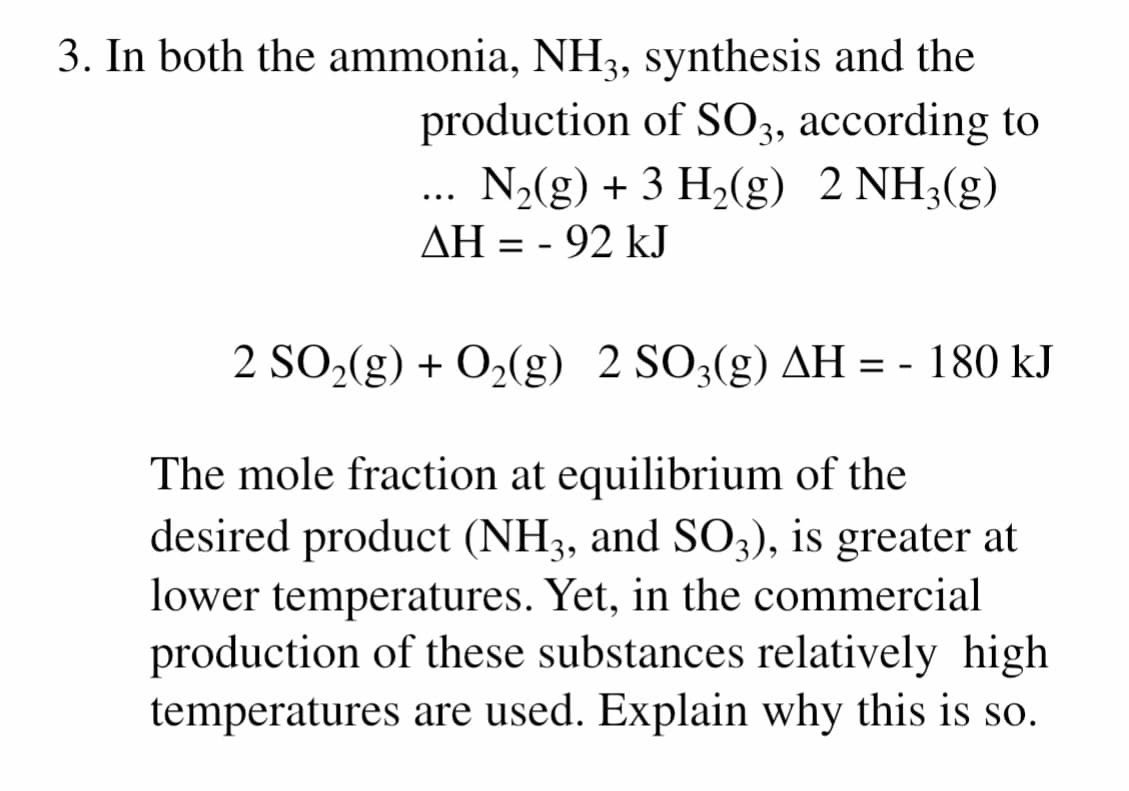
Transcribed Image Text:3. In both the ammonia, NH3, synthesis and the
production of SO3, according to
N2(g) + 3 H2(g) 2 NH3(g)
...
AH = - 92 kJ
2 SO2(g) + O2(g) 2 SO3(g) AH = - 180 kJ
The mole fraction at equilibrium of the
desired product (NH3, and SO3), is greater at
lower temperatures. Yet, in the commercial
production of these substances relatively high
temperatures are used. Explain why this is so.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT