3. The colour changes corresponding to the pH intervals for the three indicators are given in the following table:- Colour change Red to orange Yellow to blue Colourless to pink pH Interval 3 -5 Indicators Methyl orange Bromothymol blue Phenolphthalein 6 - 8 8 - 10 Comment on the end-point reached by using different indicators in Part 3.1.3 and Part 3.1.4. Confirm the suitability of each indicator by referring to the pH curves determined in this experiment.
3. The colour changes corresponding to the pH intervals for the three indicators are given in the following table:- Colour change Red to orange Yellow to blue Colourless to pink pH Interval 3 -5 Indicators Methyl orange Bromothymol blue Phenolphthalein 6 - 8 8 - 10 Comment on the end-point reached by using different indicators in Part 3.1.3 and Part 3.1.4. Confirm the suitability of each indicator by referring to the pH curves determined in this experiment.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter13: An Introduction To Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 13.11QAP: The equilibrium constant for the conjugate acid-base pair HIn+H2OH3O++In is 8.00 10-5. From the...
Related questions
Question
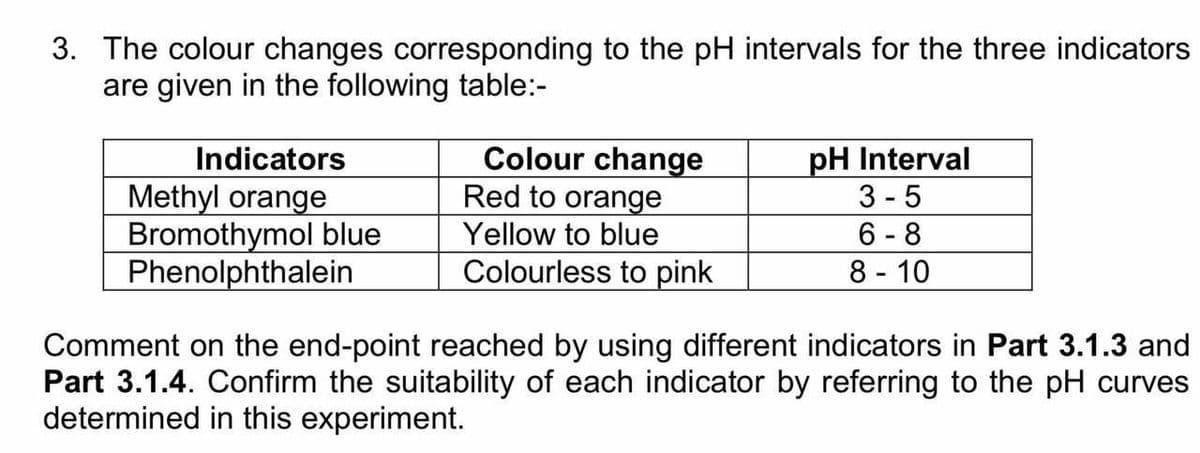
Transcribed Image Text:3. The colour changes corresponding to the pH intervals for the three indicators
are given in the following table:-
Colour change
Red to orange
pH Interval
3 - 5
6 - 8
8 - 10
Indicators
Methyl orange
Bromothymol blue
Phenolphthalein
Yellow to blue
Colourless to pink
Comment on the end-point reached by using different indicators in Part 3.1.3 and
Part 3.1.4. Confirm the suitability of each indicator by referring to the pH curves
determined in this experiment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
