PROCEDURE Acidity 100 mL aliquots of the sample with a few drops of HCI, and boil gently for a few minutes to eliminate CO2. Cool, add 3 to 4 drops of methyl red, and neutralise with 0.1 M NaOH. Introduce 2 mL of pH-10 buffer, 3 to 4 drops of Eriochrome Black T, and titrate with the prepared standard NazHzY to a colour change from red to pure, blue (Note). Report the results in terms of milligrams of CaCO, per litre water. NOTE The colour change is sluggish if Mg? is absent. In this event, add 1 to 2mL of 0.1 Mg Y2- before starting the titration (see Section 36D-4 for the preparation of this solution). Data. Mass of NazH2Y.2H20 0,9989 g Titration Initial volume (ml) final volume (ml) Volume used (ml) ? 1 0,75 25.25 2 1.25 26,75 1,75 26,87 4 2,55 27.05 1.90 21.90
PROCEDURE Acidity 100 mL aliquots of the sample with a few drops of HCI, and boil gently for a few minutes to eliminate CO2. Cool, add 3 to 4 drops of methyl red, and neutralise with 0.1 M NaOH. Introduce 2 mL of pH-10 buffer, 3 to 4 drops of Eriochrome Black T, and titrate with the prepared standard NazHzY to a colour change from red to pure, blue (Note). Report the results in terms of milligrams of CaCO, per litre water. NOTE The colour change is sluggish if Mg? is absent. In this event, add 1 to 2mL of 0.1 Mg Y2- before starting the titration (see Section 36D-4 for the preparation of this solution). Data. Mass of NazH2Y.2H20 0,9989 g Titration Initial volume (ml) final volume (ml) Volume used (ml) ? 1 0,75 25.25 2 1.25 26,75 1,75 26,87 4 2,55 27.05 1.90 21.90
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.33QAP
Related questions
Question
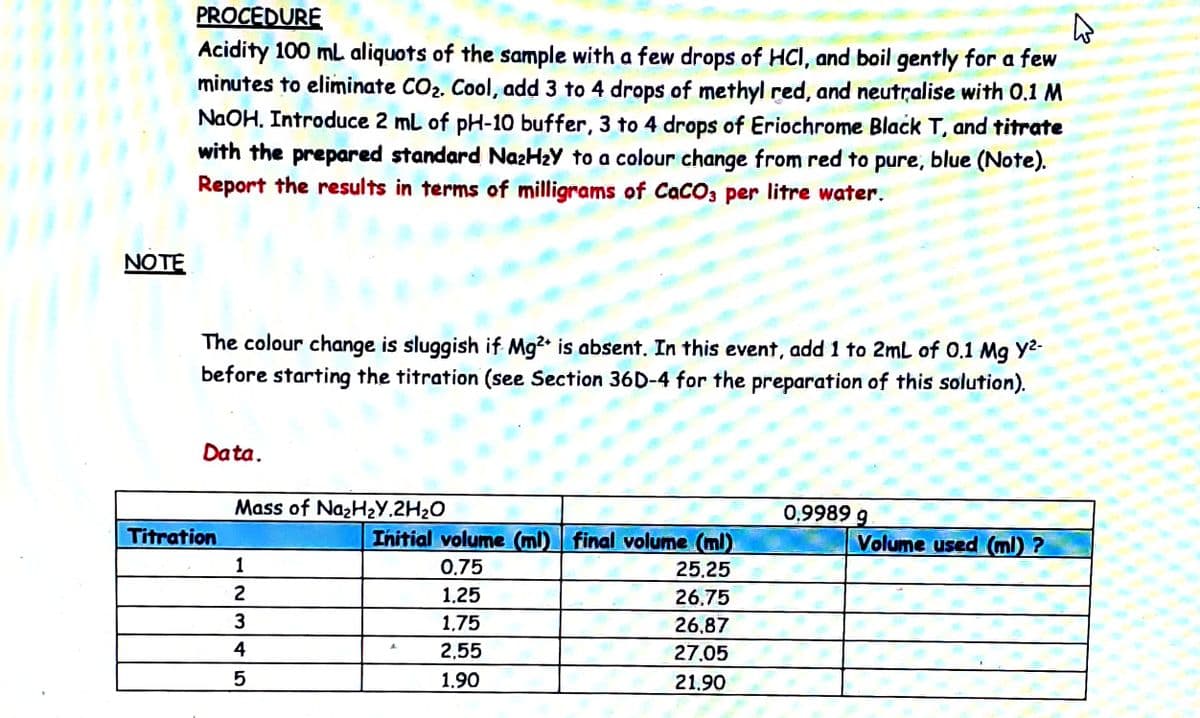
Transcribed Image Text:PROCEDURE
Acidity 100 mL aliquots of the sample with a few drops of HCI, and boil gently for a few
minutes to eliminate CO2. Cool, add 3 to 4 drops of methyl red, and neutralise with 0.1 M
NaOH. Introduce 2 ml of pH-10 buffer, 3 to 4 drops of Eriochrome Black T, and titrate
with the prepared standard NazHzY to a colour change from red to pure, blue (Note).
Report the results in terms of milligrams of CaCO; per litre water.
NOTE
The colour change is sluggish if Mg? is absent. In this event, add 1 to 2mL of 0.1 Mg Y2-
before starting the titration (see Section 36D-4 for the preparation of this solution).
Data.
Mass of NazHzY.2H2O
0,9989 g
Titration
Initial volume (ml) final volume (ml)
Volume used (ml) ?
1
0,75
25.25
2
1,25
26,75
3
1,75
26,87
4
2,55
27.05
1.90
21.90
stime
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



