3. The following mineral analysis was reported for a water sample taken from a local NJ well. Determine the total, carbonate and non-carbonate hardness in mg/L as CaCO3.
3. The following mineral analysis was reported for a water sample taken from a local NJ well. Determine the total, carbonate and non-carbonate hardness in mg/L as CaCO3.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.13QAP
Related questions
Question
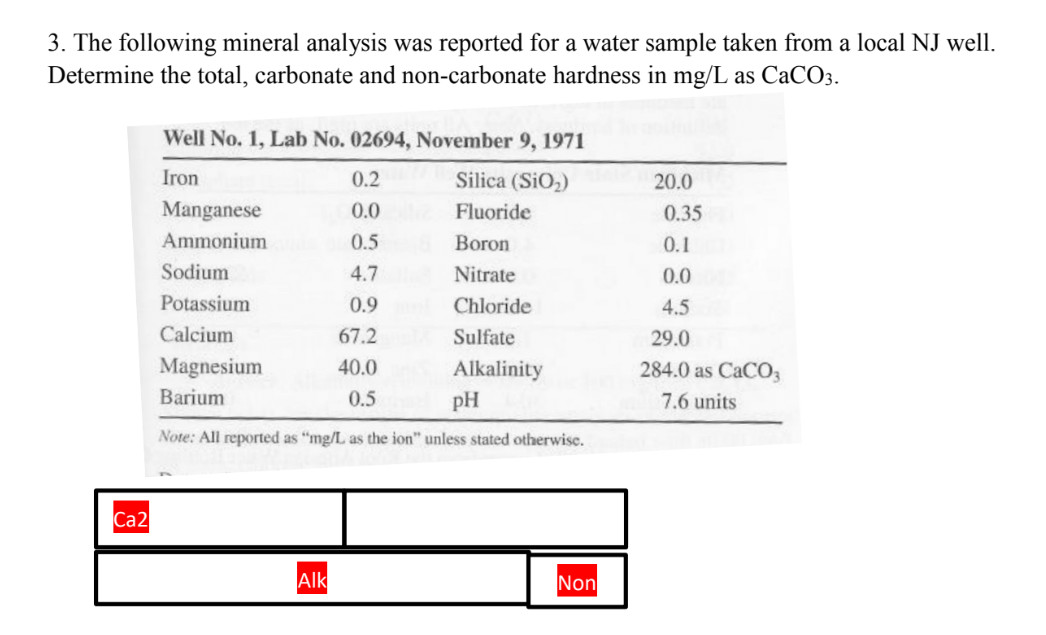
Transcribed Image Text:3. The following mineral analysis was reported for a water sample taken from a local NJ well.
Determine the total, carbonate and non-carbonate hardness in mg/L as CaCO3.
Well No. 1, Lab No. 02694, November 9, 1971
Iron
0.2
Silica (SiO2)
20.0
Manganese
0.0
Fluoride
0.35
Ammonium
0.5
Boron
0.1
Sodium
4.7
Nitrate
0.0
Potassium
0.9
Chloride
4.5
Calcium
67.2
Sulfate
29.0
Magnesium
40.0
Alkalinity
284.0 as CACO3
Barium
0.5
pH
7.6 units
Note: All reported as "mg/L as the ion" unless stated otherwise.
Ca2
Alk
Non
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning