Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter4: Gibbs Energy And Chemical Potential
Section: Chapter Questions
Problem 4.5E: Prove that the adiabatic free expansion of an ideal gas is spontaneous.
Related questions
Question
Subject is physical

Transcribed Image Text:3. Use Maxwell relations to determine an expression for the change in entropy of a van der Waals gas
with respect to a change in volume.
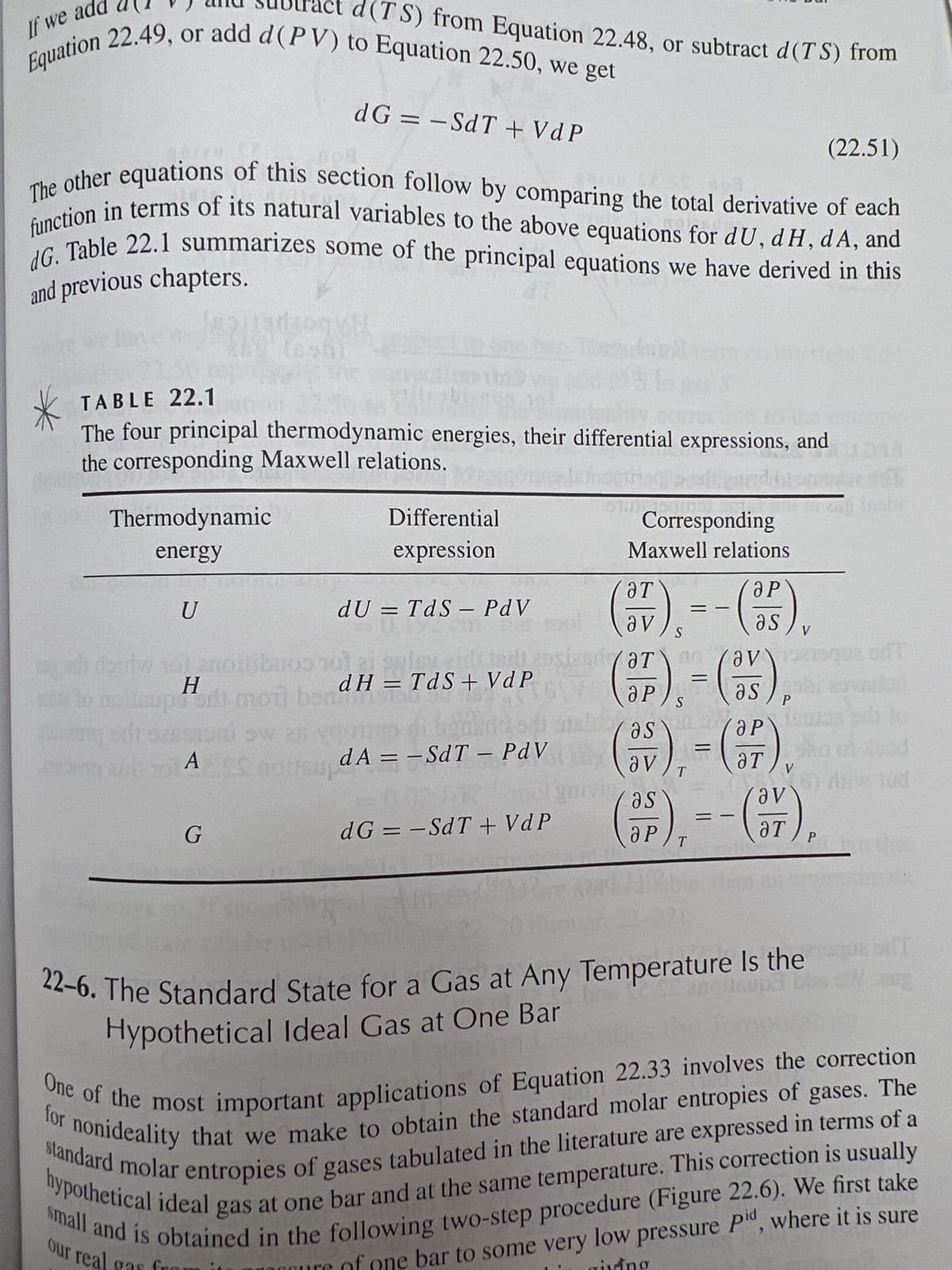
Transcribed Image Text:If we ad
Equation 22.49, or add d (PV) to Equation 22.50, we get
The other equations of this section follow by comparing the total derivative of each
function in terms of its natural variables to the above equations for dU, dH, dA, and
dG. Table 22.1 summarizes some of the principal equations we have derived in this
and previous chapters.
Thermodynamic
energy
U
TABLE 22.1
are
The four principal thermodynamic energies, their differential expressions, and
the corresponding Maxwell relations.
d(TS) from Equation 22.48, or subtract d(TS) from
Н
H____
noileupiti moit ban
onsup
og airt To A
dG = -SdT + VdP
G
Differential
expression
dU = TdS - PdV
priboo 101 zi pal
dH = TdS+ VdP
1151
dA==SdT - PdV
dG = -SdT + VdP
Corresponding
Maxwell relations
(7),=-(-35),
as
S
av
(F).-(53).
S
Ꮪ
(as),
T
a P
as ab
5/5
V
P
10
Vas
as
(P), --(57),
T
(22.51)
P
22-6. The Standard State for a Gas at Any Temperature Is the
Hypothetical Ideal Gas at One Bar
V
(a) die lud
ang fashi
ant
the
One of the most important applications of Equation 22.33 involves the correction
for nonideality that we make to obtain the standard molar entropies of gases. The
hypothetical ideal gas at one bar and at the same temperature. This correction is usually
standard molar entropies of gases tabulated in the literature are expressed in terms of a
small and is obtained in the following two-step procedure (Figure 22.6). We first take
bar to some very low pressure Pid, where it is sure
our real
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,