Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 25QAP: A sodium hydrogen carbonate-sodium carbonate buffer is to be prepared with a pH of 9.40. (a) What...
Related questions
Question
How do I complete problem 3 in a step by step?
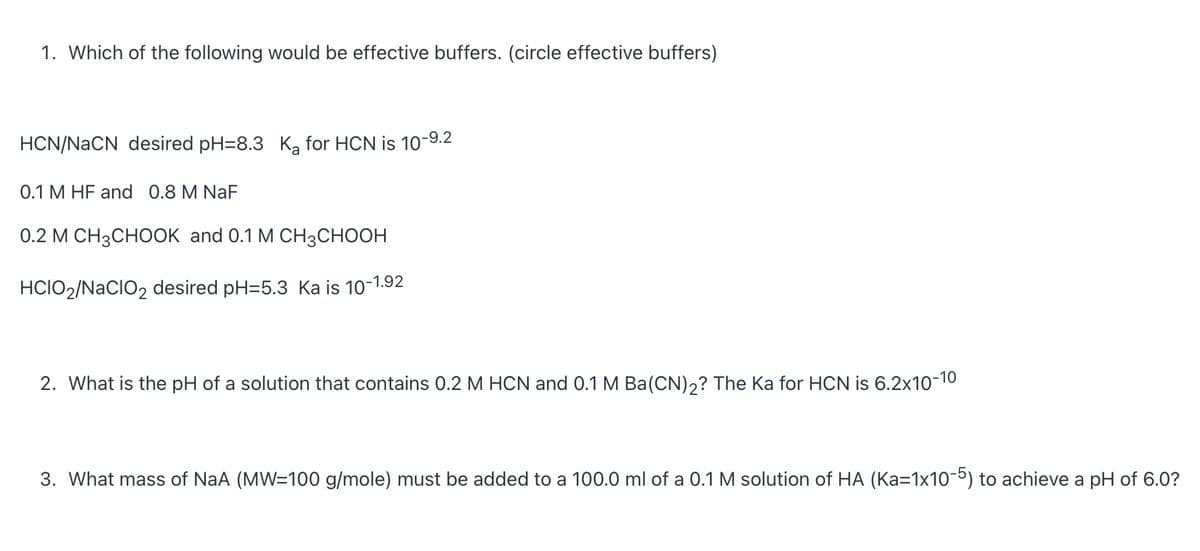
Transcribed Image Text:1. Which of the following would be effective buffers. (circle effective buffers)
HCN/NACN desired pH=8.3 K, for HCN is 10-9.2
0.1 M HF and 0.8 M NaF
0.2 М CН3СНООK and 0.1 M CHзCнОон
HCIO2/NaCIO2 desired pH=5.3 Ka is 10-1.92
2. What is the pH of a solution that contains 0.2 M HCN and 0.1 M Ba(CN)2? The Ka for HCN is 6.2x10-10
3. What mass of NaA (MW=100 g/mole) must be added to a 100.0 ml of a 0.1 M solution of HA (Ka=1x10-5) to achieve a pH of 6.0?
Expert Solution
Step 1
Buffer solution:
The solution that resists the change in pH is known as a buffer solution. The buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. It resists the pH changes when a small amount of strong acid or base is added to it.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning