Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.101PAE: 10.101 Fluorine reacts with liquid water to form gaseous hydrogen fluoride and oxygen. (a) Write a...
Related questions
Question
I need the answer for question 3

Transcribed Image Text:3
each of tne reactions observed, the reacianis or
5.
products? Explain your reasoning. (Note: the
reactants are the substances that were there
before the reaction began and the products are the
substances produced by the reaction).
2. Was energy absorbed or released in each of
the observed reactions? Explain your
reasoning.
3. Which do you think has more stored energy in
each of the reactions observed, the reactants or
products? Explain your reasoning.
4. Which do you think is more chemically stable,
the reactants or products of each of the reactions
observed? Explain your reasoning.
5. Based upon your answers to questions 3 & 4,
how does the amount of stored energy in a
substance relate to its chemical stability? Explain
your reasoning.
Transcribed Image Text:ntca.infinit..
(535) YouTube
EdClub
Math
cessibility
Last edit was 5 hours ago
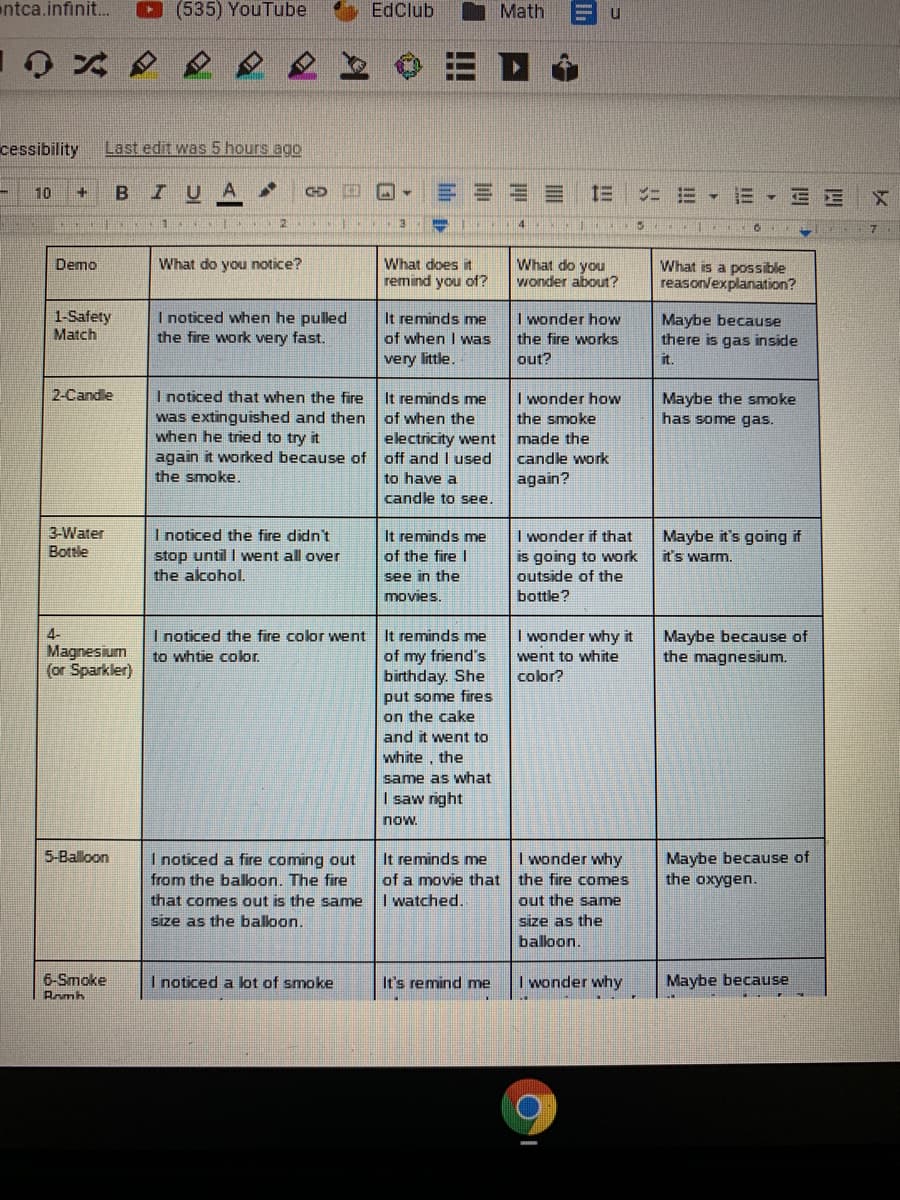
B
I U
A
==== 三
三▼三三
10
ニ 三▼
1s 1O
What does it
remind you of?
What do you
wonder about?
Demo
What do you notice?
What is a possible
reasonvexplanation?
1-Safety
Match
I noticed when he pulled
the fire work very fast.
It reminds me
of when I was
very little.
I wonder how
the fire works
Maybe because
there is gas inside
out?
it.
I noticed that when the fire
was extinguished and then of when the
when he tried to try it
again it worked because of
the smoke.
Maybe the smoke
has some gas.
2-Candle
It reminds me
I wonder how
the smoke
electricity went
off and I used
made the
candle work
to have a
again?
candle to see.
3-Water
I noticed the fire didn't
stop until I went all over
the acohol.
It reminds me
I wonder if that
is going to work
outside of the
Maybe it's going if
it's warm.
Bottle
of the fire I
see in the
movies.
bottle?
I wonder why it
4-
Magnesium
(or Sparkler)
I noticed the fire color went
to whtie color.
It reminds me
of my friend's
birthday. She
put some fires
on the cake
and it went to
Maybe because of
the magnesium.
went to white
color?
white, the
same as what
I saw right
now.
Maybe because of
the oxygen.
5-Balloon
I noticed a fire coming out
It reminds me
I wonder why
of a movie that the fire comes
I watched.
from the balloon. The fire
that comes out is the same
out the same
size as the baloon.
size as the
balloon.
6-Smoke
Rrmh
I noticed a lot of smoke
It's remind me
I wonder why
Maybe because
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
