3. Which of the following is the correct equations for the reaction of sulfur tetrafluoride? a. S + 4F --> SF4 b. 2S + 4F --> 2SF2 c. S + 2F2 --> SF4 d. S + 4F --> S + 2F2 e. SF4 --> S + 2F2 f. 2SF4 --> S2 +4F2 g. none of the above 4. What is the correct equation for the reactions of CH4 and O2? a. CH4 + O2 --> CO2 + 2H2 b. CH4 + 2O2 --> CO2 + 2H2O c. CH4 + O2 --> C + 2H2O d. CH4 + O2 --> CH4O2 e. CH4 + O2 --> CH2O + H2O f. CH4 + O2 --> C+ 2H2 + O2 g. none of the above 5. Which of the following is the correct equation for the reaction of calcium nitrate with ammonium fluoride? a. Ca(NO2)2 + 2NH4F --> CaF2 + 2NH4NO2 b. CaNO3 + 2NH4F --> CaF2 + 2NH4NO3 c. Ca(NO3)2 + 2NH4F --> 2NH4NO3 + CaF2 d. 2Ca(NO3)2 + NH4F2 --> 2CaF2 + NH4(NO3)2 e. Ca(NO3)2 + NH4F --> CaF2 + NH4NO3 f. Ca(NO3)2 + 2NH4F --> Ca(NH4)2 + 2FNO3 g. none of the above 6. Which of the following pictorially represents the following reaction: N2 + 3H2 --> 2 NH3 (Look at the picture below)
3. Which of the following is the correct equations for the reaction of sulfur tetrafluoride?
a. S + 4F --> SF4
b. 2S + 4F --> 2SF2
c. S + 2F2 --> SF4
d. S + 4F --> S + 2F2
e. SF4 --> S + 2F2
f. 2SF4 --> S2 +4F2
g. none of the above
4. What is the correct equation for the reactions of CH4 and O2?
a. CH4 + O2 --> CO2 + 2H2
b. CH4 + 2O2 --> CO2 + 2H2O
c. CH4 + O2 --> C + 2H2O
d. CH4 + O2 --> CH4O2
e. CH4 + O2 --> CH2O + H2O
f. CH4 + O2 --> C+ 2H2 + O2
g. none of the above
5. Which of the following is the correct equation for the reaction of calcium nitrate with ammonium fluoride?
a. Ca(NO2)2 + 2NH4F --> CaF2 + 2NH4NO2
b. CaNO3 + 2NH4F --> CaF2 + 2NH4NO3
c. Ca(NO3)2 + 2NH4F --> 2NH4NO3 + CaF2
d. 2Ca(NO3)2 + NH4F2 --> 2CaF2 + NH4(NO3)2
e. Ca(NO3)2 + NH4F --> CaF2 + NH4NO3
f. Ca(NO3)2 + 2NH4F --> Ca(NH4)2 + 2FNO3
g. none of the above
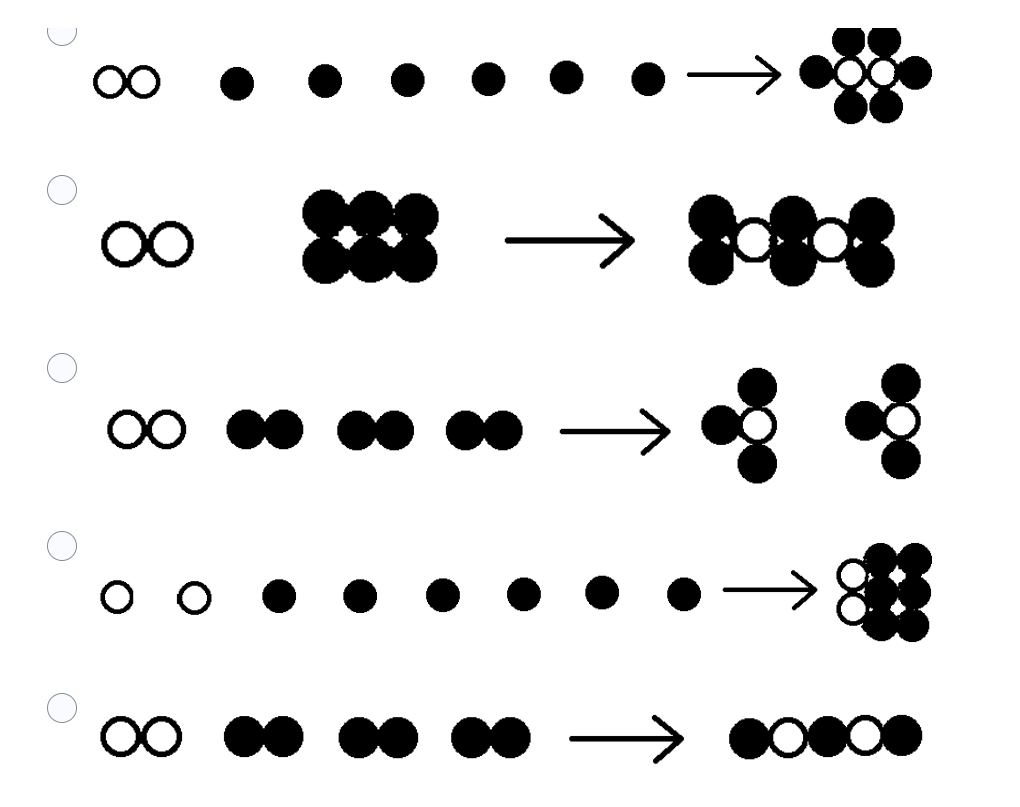
6. Which of the following pictorially represents the following reaction:
N2 + 3H2 --> 2 NH3
(Look at the picture below)
Step by step
Solved in 2 steps with 2 images


