3. You are studying an interaction between an antibody and the antigen shown below. The amine next to the benzene ring has a pKa of 10.5 and the carboxylic acid has a pKa of 4.2. Circle each of these functional groups on the molecule below. (b) NH₂ HO B. Draw the structure of the antigen at pH 7.4. pra Yantela sble OH V N 148 M3 المهم له (9) vionA EAM C. There is one ionic bond and 3 hydrogen bonds that occur between the antibody and antigen. The ionic bond forms between a glutamate on the antibody and the protonated amine of the antigen. One of the hydrogen bond forms between a serine side chain on the antibody and the carboxylic acid of the antigen. Draw the ionic bond, including the full chemical structure of the side chain of the antibody. Indicate the ionic bond with a wavy line. D. A mutation occurs in the antibody such that the serine described above changes to an alanine. Do you expect the antigen to bind more or less tightly in the mutant compared to the original? Briefly explain.
3. You are studying an interaction between an antibody and the antigen shown below. The amine next to the benzene ring has a pKa of 10.5 and the carboxylic acid has a pKa of 4.2. Circle each of these functional groups on the molecule below. (b) NH₂ HO B. Draw the structure of the antigen at pH 7.4. pra Yantela sble OH V N 148 M3 المهم له (9) vionA EAM C. There is one ionic bond and 3 hydrogen bonds that occur between the antibody and antigen. The ionic bond forms between a glutamate on the antibody and the protonated amine of the antigen. One of the hydrogen bond forms between a serine side chain on the antibody and the carboxylic acid of the antigen. Draw the ionic bond, including the full chemical structure of the side chain of the antibody. Indicate the ionic bond with a wavy line. D. A mutation occurs in the antibody such that the serine described above changes to an alanine. Do you expect the antigen to bind more or less tightly in the mutant compared to the original? Briefly explain.
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter45: Defenses Against Disease
Section: Chapter Questions
Problem 5TYK
Related questions
Question
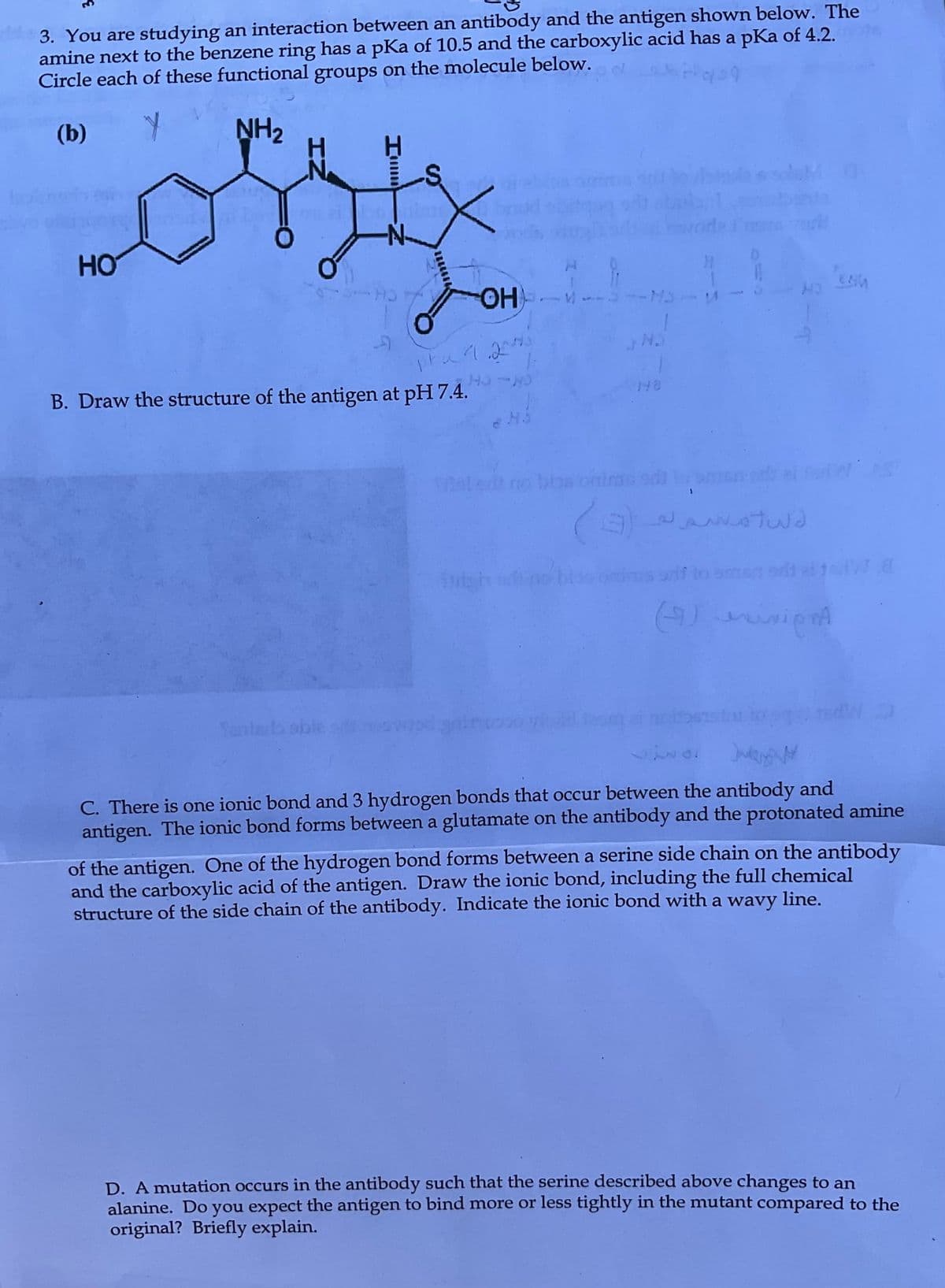
Transcribed Image Text:3. You are studying an interaction between an antibody and the antigen shown below. The
amine next to the benzene ring has a pKa of 10.5 and the carboxylic acid has a pKa of 4.2.
Circle each of these functional groups on the molecule below.
(b)
Y NH₂
HO
B. Draw the structure of the antigen at pH 7.4.
Santeita oble
OHM-
pra 120773
1111
Suish
MS
HJ
HO
ng bbsonings sd
(3) ~
misn
11
ال مام له
Nor
Tesn
prifto emen
(JurisionA
WAS
Wa
Judas
C. There is one ionic bond and 3 hydrogen bonds that occur between the antibody and
antigen. The ionic bond forms between a glutamate on the antibody and the protonated amine
of the antigen. One of the hydrogen bond forms between a serine side chain on the antibody
and the carboxylic acid of the antigen. Draw the ionic bond, including the full chemical
structure of the side chain of the antibody. Indicate the ionic bond with a wavy line.
D. A mutation occurs in the antibody such that the serine described above changes to an
alanine. Do you expect the antigen to bind more or less tightly in the mutant compared to the
original? Briefly explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 6 images

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage