3. You see a gold ring in a flea market that you'd like to have, and the price seems right, so you buy it. On the drive home you wonder if it's really gold. You do a quick experiment and find it takes 38.7 J of heat to raise the temperature of the 6.00 g ring from 25°C to 75°C. You know that the specific heat of gold is 0.128 J/g°C; is your ring really made of gold?
3. You see a gold ring in a flea market that you'd like to have, and the price seems right, so you buy it. On the drive home you wonder if it's really gold. You do a quick experiment and find it takes 38.7 J of heat to raise the temperature of the 6.00 g ring from 25°C to 75°C. You know that the specific heat of gold is 0.128 J/g°C; is your ring really made of gold?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
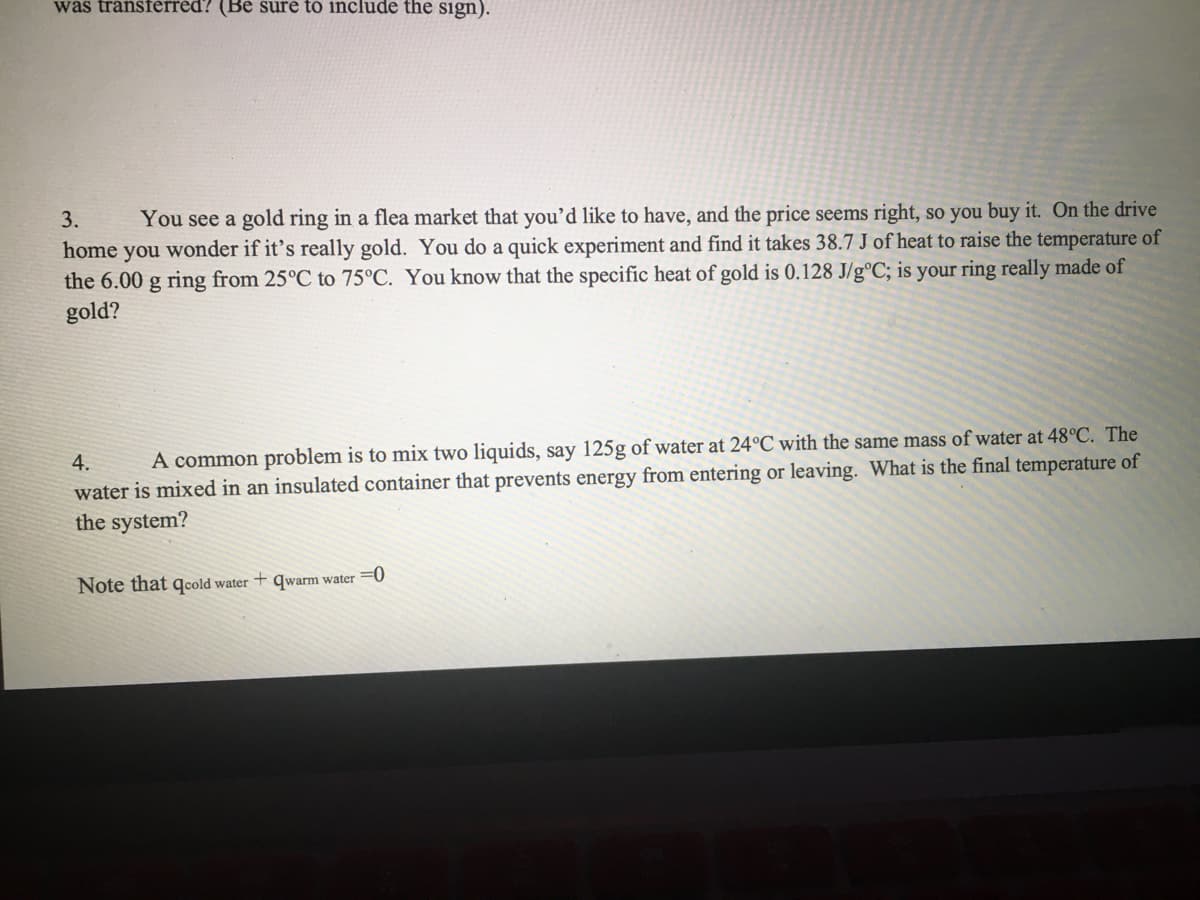
Transcribed Image Text:was tránsférréd? (Be sure to include the sign).
You see a gold ring in a flea market that you'd like to have, and the price seems right, so you buy it. On the drive
wonder if it's really gold. You do a quick experiment and find it takes 38.7 J of heat to raise the temperature of
the 6.00 g ring from 25°C to 75°C. You know that the specific heat of gold is 0.128 J/g°C; is your ring really made of
3.
home
you
gold?
4.
A common problem is to mix two liquids, say 125g of water at 24°C with the same mass of water at 48°C. The
water is mixed in an insulated container that prevents energy from entering or leaving. What is the final temperature of
the system?
Note that qcold water + qwarm water =0
Expert Solution
Step 1
"Since you have asked multiple question, we will solve the first question for you. If you
want any specific question to be solved then please specify the question number or post
only that question.”
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning