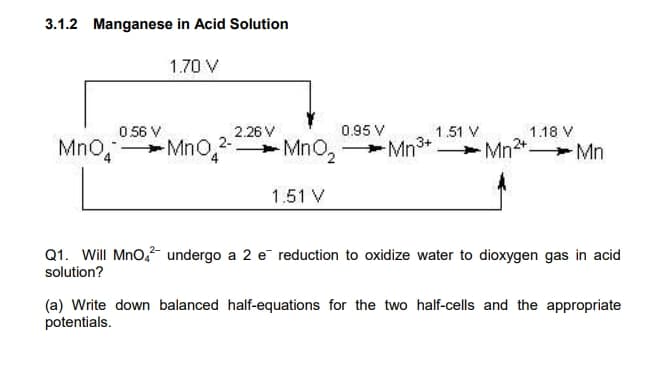
3.1.2 Manganese in Acid Solution 1.70 V 0 56 V 2.26 V 0.95 V 1.51 V 1.18 V Mno, -Mno,2- MnO, MnO, Mn° + Mn2+ Mn 1.51 V Q1. Will MnO,- undergo a 2 e reduction to oxidize water to dioxygen gas in acid solution? (a) Write down balanced half-equations for the two half-cells and the appropriate potentials.
Q: If electrolysis of aqueous solution of H₂SO4 to form per disulphuric acid (H₂S2O8). O₂ and H₂ are…
A:
Q: An electrochemical cell consist of 0.2715 lbs of MnO2 and 0.2524 lbs of PbO2. A current flow…
A: In the given question we have to calculate the series of questions for electrochemical cell,
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Given: Current = 7.33 A Time passed = 10 hour Power = 0.2229 KWh. Current efficiency = 81 %
Q: A current is passed through 500.0 mL of a solution of Cal2. The following electrode reactions occur:…
A: Hi, as you have posted multiple subparts in a single question and have not mentioned which subparts…
Q: Calculate the Ecell of the following cell: Anode: solid cadmium immersed in the saturated solution…
A: The standard cell potential, temperature, reaction quotient, and the cell potential of an…
Q: Due to COVID-19 coupled with erratic power supply from ZESCO, you decide to play around with…
A: As you have asked multipart questions, we have answered the first 3 first of your question. If you…
Q: An electrolysis cell containing MSO4solution is operated for 1.0 hr at constant current of 0.200 A.…
A:
Q: A certaln metal M forms a soluble sulfate salt M,S0,. Suppose the left half cell of a galvanic cell…
A: The Nernst equation is having a formula, Ecell=Ecell°-2.303RTnFlogQ Ecell is the potential in volts.…
Q: 2. In an industrial atmosphere with high humid- 2. Referring to the galvanic series of some com- ity…
A: According to Q&A guidelines of Bartleby, I am allowed to answer only one question out of…
Q: Telephone Number: 7738-17 Email: tpchrdnova@gmail.com NAME: GRADE & SECTION: SUBJECT TEACHER:…
A:
Q: Study the redox reactions below. For each, identify and write the half-reaction that occurs in the…
A: a) The reaction taking place is given as, => Li (s) + F2 (g) ------> Li+ (aq) + 2 F- (aq)
Q: Q2 (a) What is the IUPAC name of C4H8? Provide brief explanation. (b) Differentiate between weak and…
A:
Q: A concentration cell was made using a Zn metal electrode in a saturated solution of ZNCO3 for the…
A: Given data,Zn2+(cathode)=1.00MEMF=0.148VTemperature=25oC
Q: 1. Thirty minutes (30 m) of electrolysis of a solution of CuSO4 produced 3.75 g of Cu at the…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Cr* + 3e --> Cr(s) E =-0.744 V Ni2* + 2e --> Ni(s) E =-0.236 V a. Which electrode increases in mass…
A:
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Given: Current = 5.72 A Time passed = 8 hour Power = 0.2293 KWh. Current efficiency = 91 %
Q: A steel coupon with an anode surface area of 1000 cm is placed in an electrolyte. The corrosion…
A: Electrochemical equivalent is the amount of element deposited at an electrode when one ampere of…
Q: Due to COVID-19 coupled with erratic power supply from ZESCO, you decide to play around with…
A:
Q: For the galvanic (voltaic) cell Zn(s) | Zn2 (aq) I| Cd2-(aq) | Cd(s) E° is 0.36 V at 25°GC 9aWhat is…
A: The relation between equilibrium constant and standard cell potential is shown by equation (1), in…
Q: An electrolysis cell containing MSO4 solution is operated for 1.0 h at a constant current of 0.200…
A: Given, An electrolysis of MSO4 solution for 1.0 h at a constant current of 0.200 A. If the current…
Q: 1. The galvanizing of iron sheet can be carried electrolytically using a bath containing zinc…
A: Galvanizing is a process where iron is coated with zinc which provide protection against corrosion…
Q: A dilute NaNO; solution is to be electrolyzed with a pair of Pt electrode at a current density of…
A: Principle:A spectrophotometric determination using a reaction between nitrite,suphanilamide and…
Q: It is desired to deposit 0.87 g of iron (MW = 55.85) from a FeCl3 solution by the passage of 2.5 A…
A: Given mass of of iron = 0.87 g MW for iron = 55.85 Current = 2.5 A Efficiency 85%
Q: A H, fuel cell generates 4A current output, cell reaction: H,(g) + ½O,(g) → H,O(g) (1) What is the…
A: Answer: Given Fuel cell reaction is: H2(g)+12O2(g)→H2O(g)
Q: Consider the electrochemical cell, Ni (s) | NiCl2 (aq, 0.0050 mol kg − 1) | Hg2Cl2 (s) | Hg (l), for…
A: Since you have posted a question with multiple sub-parts, we will solve the first three for you. To…
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Electrical energy consumed = 0.2018 kWh.
Q: Suppose the Zn|Zn²+ || MnO,|Mn²+ cell from Example 17.5 is operated at pH 2.00 with [MnO,] = 0.12 M,…
A: Nernst equation The equation that relates the cell potential with the concentration is called Nernst…
Q: 2. One cell compartment is comprised of an Sc electrode in a 5.04 x 10 M solution of Sc at T= 347.3…
A: Q-2: Given: [Sc3+]=5.04 x 10-4 M Temperature, T=347.3 K Ecell=?
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Answer: Given current efficiency is 91%, therefore actual current passed…
Q: Consider a hypothetical salt of low solubility, "MX", where M+ is a metal cation and X- is a…
A: For the cell, reduction takes place at cathode and oxidation takes place at anode. Thus, the…
Q: The corrosion potential of mild steel in a deaerated solution of pH = 2 is -0.64 V versus saturated…
A: Steel is an alloy of mainly iron and carbon, with iron being the major constituent. So, we'll…
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Given: Current = 6.2 A Time passed = 11 hour Power = 0.2406 KWh. Current efficiency = 96 %
Q: The electromotive force of the Pb(cr.)/ pbSO4( cr.) / Na₂SO4.10H₂O (sat.) / Hg₂SO4 (cr) / Hg (I.) is…
A: Ecell = 0.9647 V (Ecell)/ (T) = 1.74 x 10-4
Q: SIiver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) +e-…
A: According to the question, we need to calculate the mass of silver deposited when current of 6.4…
Q: Halogen lightbulb filaments are sometimes made from a high melting point metal. The most common ore…
A: Given :- Current Passed = I = 74.00 A = 74.00 C/s Time = t = 709.2 minutes = 42552 s Metal M…
Q: Calculate the required voltage (V) if an unknown metal (M) was electrodeposited from an aqueous…
A: Given: Current = 4.1 A Time passed = 9 hour Power = 0.2027 KWh. Current efficiency = 93 %
Q: 5b) Óxidation of lactate to pyruvate by cytochrome C (representing as cytochrome C(Fe³)) is an…
A: Given Pyruvate + 2H+ + 2e- →Lactate-…
Q: 27. What is the reduction potential for the half-reaction at 25°c Al3+ + 3e- → AlI, if [AI3*] =…
A: What is the reduction potential for the half reaction at 250C Al3+ + 3e-→Al, If [Al3+] = 0.10 M…
Q: The galvanizing of the iron sheet can be carried electrolytically using a bath containing zinc…
A: Given: Dimensions of iron sheet = 2.0 m wide and 80m long Galvanizing thickness= 0.49 mm density of…
Q: The cell in Figure could be used to provide a measureof the pH in the cathode half-cell. Calculate…
A: In the given cell, Cathode (Reduction half): 2H+(aq) + 2e- → H2(g)Anode (Oxidation half): Zn(s) →…
Q: . In a galvanic cell, one half-cell consists of a zinc strip dipped into a 1.00 M solution of…
A: Given data: Cell potential = 0.425 V Standard electrode potential for zinc half cell = -0.763 V…
Q: Like any piece of apparatus, an electrolytic cell operates atless than 100% efficiency. A cell…
A: Current efficiency is the ratio of mass deposited to the theoretical mass deposited calculated by…
Q: A Zn/air battery contains a Zn plate immersed in 0.644 M ZnCl2 at one ele and air passing over a…
A: pplying the Nernst equation to a simple electrochemical cell such as the Zn/Cu cell allows us to see…
Q: d) Prove that for two electrodes of the same metal coupled and immersed in electrolytes of…
A: #(d): In the given Galvanic cell, the electrode is the same in both the half-cells. But the…
Q: PbSO4
A: Pb(s) + PbO2(s) + 2SO42-(aq) + 4H+ = 2PbSO4(s) + 2H2O Current = 6 A Volt = 12 V Time = 1 h 22…
Q: harrivtry (b) Draw and explain the cyclic voltammo- gram of Fe(n5-C5H5)2 in CH₂Cl₂, where [NBu4]…
A: The correct answer is given below
Q: 6. The corrosion potential of mild steel in a deaerated solution of pH = 2 is -0.64 V versus…
A: The reactions that occur in the given cell are stated below:
Q: . Consider a galvanic cell with the anode containing a Zn metal electrode and a 0.10 M…
A: Reduction half cell reaction : Zn2+(aq)+2e- --------------> Zn(s) E0 cell = -0.79V…
Q: Chromium plating is applied by electrolysis to objects suspended in a dichromate solution, according…
A: GivenCr2O72- + H+ + e- → Cr(s) + H2O (unbalanced)Surfacre area = 2500 cm2Thickness =…
Q: What is the forward rate constant (k) for a single electron reduction at an electrode set to 0.35V…
A:
Step by step
Solved in 2 steps

- The capacity of batteries such as the typical AA alkalinebattery is expressed in units of milliamp-hours (mAh). AnAA alkaline battery yields a nominal capacity of 2850 mAh.(a) What quantity of interest to the consumer is beingexpressed by the units of mAh? (b) The starting voltage of afresh alkaline battery is 1.55 V. The voltage decreases duringdischarge and is 0.80 V when the battery has delivered itsrated capacity. If we assume that the voltage declines linearlyas current is withdrawn, estimate the total maximumelectrical work the battery could perform during discharge.2. A batch of parts is plated with copper in an electrolytic bath running at 0.15V forexactly 2 hours. What is the energy cost if the electric utility charges company$ 0.05 per kWh? (provide the given and required to find in the problem) (Kindly provide a CLEAR and COMPLETE solution) (Answer should be typewritten)In the electrolysis of water, how long will it take to produce 1.000 × 102 L of H2 at STP (273 K and 1.00 bar) using an electrolytic cell through which a current of 58.50 mA flows? (Answer in Years please!)
- Oxidation of hydrogen gas to water happens at the anode, while reduction of oxygen gas to hydroxide happens at the cathode. A pure hydrogen at the anode side at 80 kPa and uses air at 1 bar as the oxygen source in the cathode side. At a temperature of 30oC. Assuming no side reactions due to the existence of nitrogen in the cathode, answer the following: a. What is the overall reaction? How is the overall reaction it different from hydrogen combustion?b) Determine the standard cell potential, Eocellc) Determine the actual cell potential, EcellOxidation of hydrogen gas to water happens at the anode, while reduction of oxygen gas to hydroxide happens at the cathode. A pure hydrogen at the anode side at 80 kPa and uses air at 1 bar as the oxygen source in the cathode side. At a temperature of 30oC. Assuming no side reactions due to the existence of nitrogen in the cathode, answer the following: a. What is the overall reaction? How is the overall reaction it different from hydrogen combustion?b) Determine the standard cell potential, Eocellc) Determine the actual cell potential, Ecelld) What can you say about the spontaneity of the actual fuel cell reaction at low temperatures? Will spontaneity decrease or increase at lower temperatures?. Consider a galvanic cell with the anode containing a Zn metal electrode and a 0.10 M Zn(NO3)2solution and the other half-cell containing a Sn metal electrode and a 0.10 M Sn(NO3)2 solution. If themeasured Ecell value is +650 mV and the Zn2+/Zn reduction potential is assumed to be −790 mV, what isthe Sn2+/Sn reduction potential? Show all work.
- It is desired to assemble a concentration cell with a potential of 120 mV withtwo Zn(s) electrodes immersed, each one, in a ZnSO4 solution. What should be theratio between Zn2+ activities in the two electrodes to reach the desired potential?2) If 25g of NaCl is dissolved into a final volume of 500mL, what is the percent (w/v) concentration of NaCl in the solution? COMPUTE 3) How will you prepare 200mL of 0.3M NaCl prepared? COMPUTE and Include a short instruction in the preparation. 4) From a stock solution of 1M Tris solution, how will you prepare 400mL of 0.2M? COMPUTE and Do not forget to include a short instruction in the preparation. 5) How is 4mL of 50mM NaCl solution be prepared from a 2M NaCl stock? COMPUTE and Do not forget to include a short instruction in the preparation. 6) What is the molar concentration of a 10% NaCl solution? (At 25⁰C, the density of 10% NaCl is 1.09 g/ml ) COMPUTEArsenic(III) sulfide is oxidized with concentrated nitric acid into arsenic acid and sulfuric acid, with release of nitrogen dioxide gas. Compile the balanced chemical eqn for this reaction using oxidation-reduction electron-ion half reaction method (must show explicitly your work). The sum of all stochiometric coefficients in this chemical eqn is: A 30 B 50 C 70 D None of those is correct. If so, what is your value?
- Jansen Gas creates three types of aviation gasoline(avgas), labeled A, B, and C. It does this by blendingfour feedstocks: Alkylate; Catalytic CrackedGasoline; Straight Run Gasoline; and Isopentane.Jansen’s production manager, Dave Wagner, hascompiled the data on feedstocks and gas types inTables 4.6 and 4.7. Table 4.6 lists the availabilitiesand values of the feedstocks, as well as their keychemical properties, Reid vapor pressure, and octanerating. Table 4.7 lists the gallons required, theprices, and chemical requirements of the three gastypes. Table 4.6 Data on Feedstocks Feedstock Alkylate CCG SRG Isopentane Gallons available (1000s) 140 130 140 110 Value per gallon $4.50 $2.50 $2.25 $2.35 Reid vapor pressure 5 8 4 20 Octane (low TEL) 98 87 83 101 Octane (high TEL) 107 93 89 108 Table 4.7 Data on Gasoline Gasoline A B C Gallons required (1000s) 120 130 120 Price per gallon $3.00 $3.50 $4.00 Max Reid pressure 7 7 7 Min octane 90 97 100 TEL level Low High High Note that each feedstock…Q/ ture or false 1- The difference between wet and dry corrosion, in the first case it occurs in the presence of moisture and the second in its absence * 2- It is necessary to fix the names of the equipment on the illustrative curve of the industrial process * 3- The flow curves are classified according to the number of reactors and the mechanism of separation or non-separation of the products * 4- In the processes that involve the purification of raw materials and products, it is preferable to purify the raw materials by adsorption, while the products are purified by washing, stripping and absorption. 5- The amount of dissolved substances in well water differs greatly from surface water due to the difference in temperature. * 6- The biological waste has no significant effect on the efficiency of the flow, but rather its effect on the toxicity of the water used for industrial purposes. 7- Several methods are used to remove unacceptable odors from water, including chemical…Angeline performed an experiment on vitamin C content. She purchased bell peppers of different colours from local wet market. Bell pepper samples were cut, blended and homogenized with a fixed portion of water. Subsequently, coulometric titration was performed using iodine under constant current of 60 mA. In one of the experiment replicate,starch end point was achieved after 18 minutes. Suppose that bell pepper sample recorded initial mass of 115 g, deduce it’s vitamin C (176 g/mol) content in percentage. (F = 96500C)

