3.72 Fermentation is a complex chemical process of wine making in which glucose is converted into ethanol and carbon dioxide: C6H1206 – 2C2H5OH + 2CO2 glucose ethanol Starting with 500.4 g of glucose, what is the maxi- mum amount of ethanol in grams and in liters that can be obtained by this process? (Density of ethanol = 0.789 a/mL.)
3.72 Fermentation is a complex chemical process of wine making in which glucose is converted into ethanol and carbon dioxide: C6H1206 – 2C2H5OH + 2CO2 glucose ethanol Starting with 500.4 g of glucose, what is the maxi- mum amount of ethanol in grams and in liters that can be obtained by this process? (Density of ethanol = 0.789 a/mL.)
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 47A
Related questions
Question
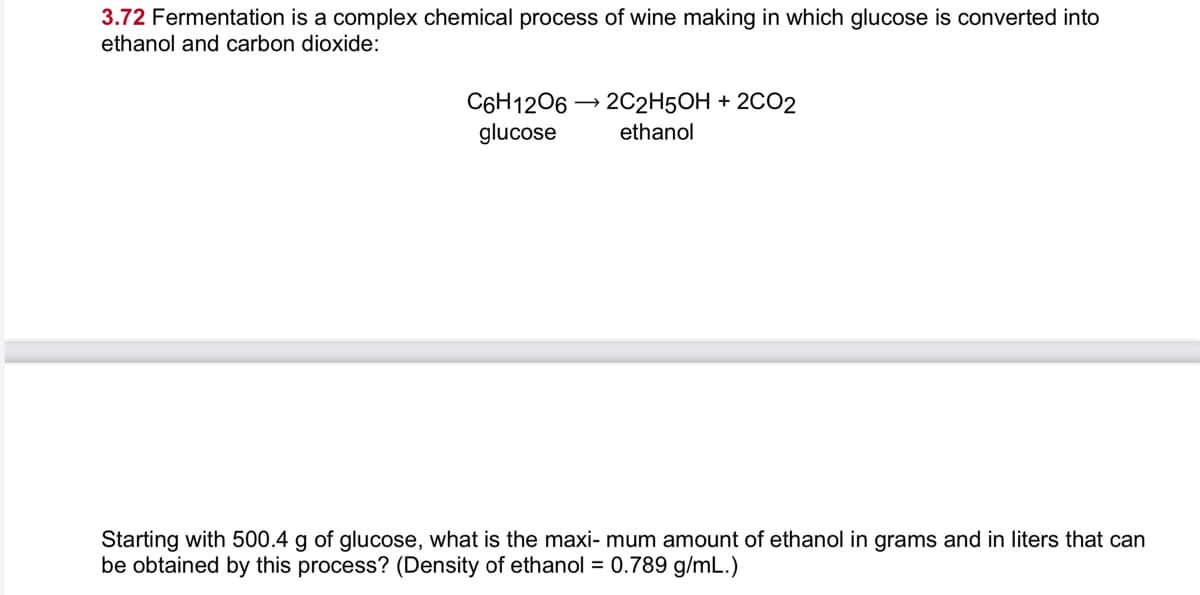
Transcribed Image Text:3.72 Fermentation is a complex chemical process of wine making in which glucose is converted into
ethanol and carbon dioxide:
C6H1206 → 202H5OH + 2CO2
glucose
ethanol
Starting with 500.4 g of glucose, what is the maxi- mum amount of ethanol in grams and in liters that can
be obtained by this process? (Density of ethanol = 0.789 g/mL.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div