3.77 g of potassium is placed in 375 mL of 0.850 mol/L chromium (III) nitrate solution as follows 3 K (s) + Cr(NO3)3 (aq) D 3 KNO3 (aq) + Cr (s) Determine which reactant is the limiting reagent and calculate the mass, in grams, of Cr produced.
3.77 g of potassium is placed in 375 mL of 0.850 mol/L chromium (III) nitrate solution as follows 3 K (s) + Cr(NO3)3 (aq) D 3 KNO3 (aq) + Cr (s) Determine which reactant is the limiting reagent and calculate the mass, in grams, of Cr produced.
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.18QAP
Related questions
Question
All information listed in question find the limiting reagents in grams.
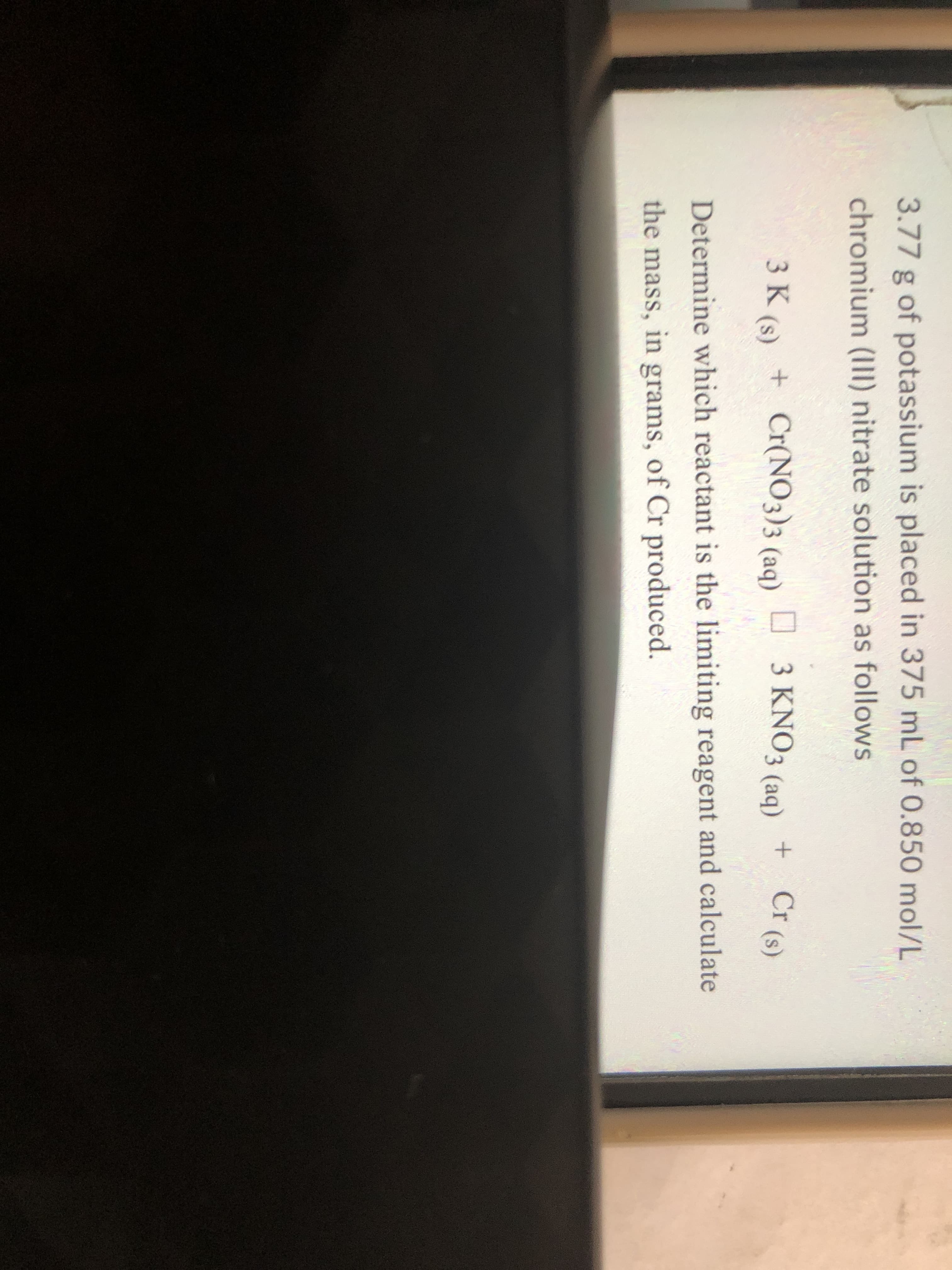
Transcribed Image Text:3.77 g of potassium is placed in 375 mL of 0.850 mol/L
chromium (III) nitrate solution as follows
3 K (s) + Cr(NO3)3 (aq) D 3 KNO3 (aq)
+ Cr (s)
Determine which reactant is the limiting reagent and calculate
the mass, in grams, of Cr produced.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning