One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(I) hydroxide from a solution containing rhodium() sulfate according to the following balanced chemical equation: Rh(SOah(aq) + 6N2OH(aq) - 2Rh(OH),(s) + 3Na,So,(aq) What is the theoretical yield of rhodium(Il) hydroxide from the reaction of 0.500 g of thodium(Il) sulfate with 1.100 g of sodium hydroxide? O 0.500 g O 0.156 g O 0.312 g O 141 g O 1.60 g
One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(I) hydroxide from a solution containing rhodium() sulfate according to the following balanced chemical equation: Rh(SOah(aq) + 6N2OH(aq) - 2Rh(OH),(s) + 3Na,So,(aq) What is the theoretical yield of rhodium(Il) hydroxide from the reaction of 0.500 g of thodium(Il) sulfate with 1.100 g of sodium hydroxide? O 0.500 g O 0.156 g O 0.312 g O 141 g O 1.60 g
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 78QAP: When 85.0 mL of 0.250 M Ba(OH)2 solution is added to 85.00 mL of 0.250 M Al (NO3)3 solution, a white...
Related questions
Question

Transcribed Image Text:One step in the isolation of pure rhodium metal (Rh) is the precipitation of rhodium(I) hydroxide from a solution containing rhodium(I) sulfate according to the following balanced
chemical equation:
Rh(SOab(aq) + 6N2OH(aq) - 2Rh(OH),(s) + 3Na, so(aq)
What is the theoretical yielid of rhodium(II) hydroxide from the reaction of 0.500 g of thodium(I) sulfate with 1.100 g of sodium hydroxide?
O 0.500 g
O 0.156 g
O 0.312 g
O 1.41 g
O 1.60 g
If 48.0 g of O, is mixed with 48.0 g of H2 and the mixture is ignited, what is the maximum mass of water that may be produced?
O 48.0 g
O 85.3 g
O 54.0 g
O 96 g
432 g
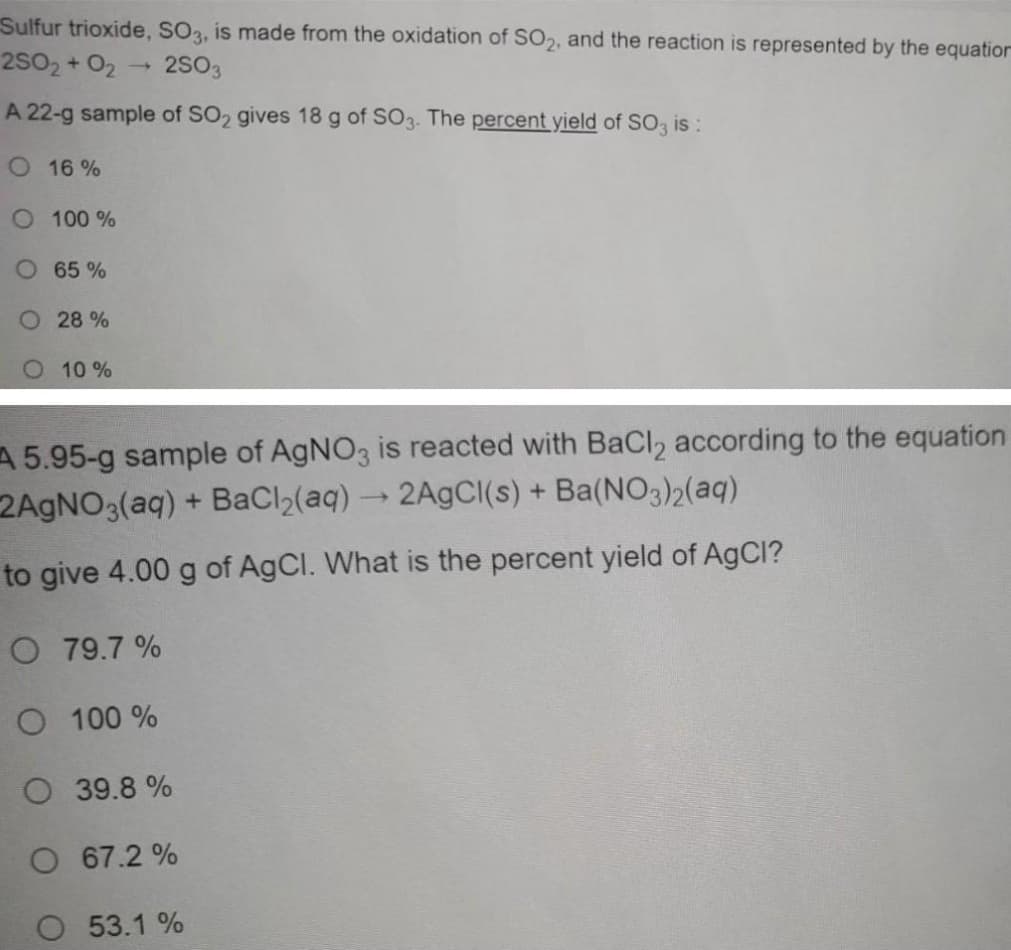
Transcribed Image Text:Sulfur trioxide, SO3, is made from the oxidation of SO2, and the reaction is represented by the equation
2S02 + O2
2S03
A 22-g sample of SO2 gives 18 g of SO3. The percent yield of SO3 is:
O 16 %
O 100 %
O 65%
O 28 %
O 10%
A 5.95-g sample of AgNO3 is reacted with BaCl2 according to the equation
2AGNO3(aq) + BaCl2(aq) →2A9CII(s) + Ba(NO3)2(aq)
to give 4.00 g of AgCl. What is the percent yield of AgCl?
O 79.7 %
O 100 %
O 39.8 %
O 67.2 %
53.1 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
