38. What is the total number of grams of KCI (molar mass = 74.6g/mol) in 1.00 L of 0.200 molar solution? %3D a. 7.46 O D. 14.9 O c. 22.4 O d 29.8
38. What is the total number of grams of KCI (molar mass = 74.6g/mol) in 1.00 L of 0.200 molar solution? %3D a. 7.46 O D. 14.9 O c. 22.4 O d 29.8
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.7QAP
Related questions
Question
answer 38
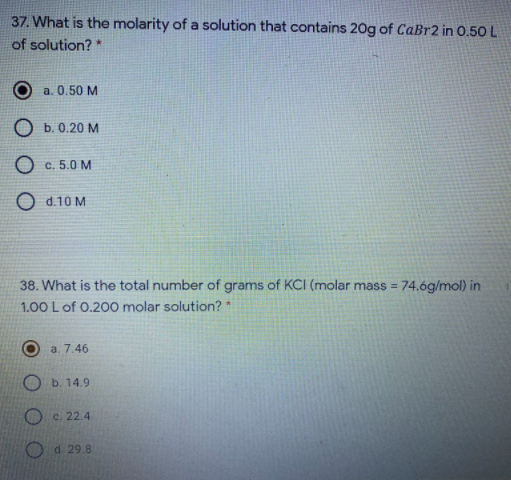
Transcribed Image Text:37. What is the molarity of a solution that contains 20g of CaBr2 in 0.50 L
of solution? *
a. 0.50 M
O b. 0.20 M
O c. 5.0 M
O d.10 M
38. What is the total number of grams of KCI (molar mass = 74.6g/mol) in
%3D
1.00 L of 0.200 molar solution? *
a. 7.46
O b. 14.9
O c. 22.4
O d 29.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning