38. Which of the following statements about the solubility of organic compounds in H2O is true? A) The non-polar part of a molecule that is not attracted to water is said to be hydrophilic. B) The non-polar part of a molecule that is not attracted to water is said to be hydrophobic. C) The polar part of a molecule that can hydrogen bond to water is said to be hydrophobic. D) For an organic compound with one functional group that contains an O or N atom, the compound is water soluble only if it has 2 5 carbons.
38. Which of the following statements about the solubility of organic compounds in H2O is true? A) The non-polar part of a molecule that is not attracted to water is said to be hydrophilic. B) The non-polar part of a molecule that is not attracted to water is said to be hydrophobic. C) The polar part of a molecule that can hydrogen bond to water is said to be hydrophobic. D) For an organic compound with one functional group that contains an O or N atom, the compound is water soluble only if it has 2 5 carbons.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.44QE: Predict the relative solubility of each compound in the two solvents, on the basis of intermolecular...
Related questions
Question
answer 38
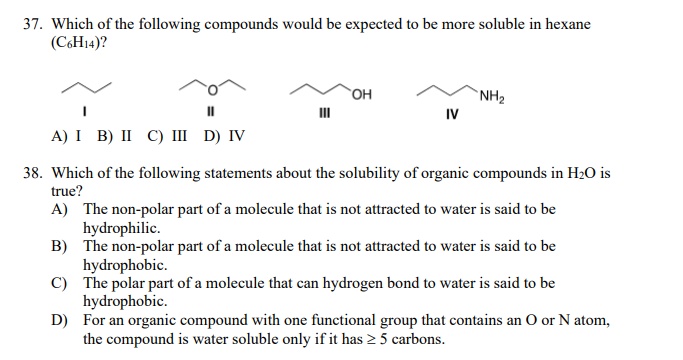
Transcribed Image Text:37. Which of the following compounds would be expected to be more soluble in hexane
(C6H14)?
`OH
`NH2
IV
A) I B) II С) Ш D) V
38. Which of the following statements about the solubility of organic compounds in H2O is
true?
A) The non-polar part of a molecule that is not attracted to water is said to be
hydrophilic.
B) The non-polar part of a molecule that is not attracted to water is said to be
hydrophobic.
C) The polar part of a molecule that can hydrogen bond to water is said to be
hydrophobic.
D) For an organic compound with one functional group that contains an O or N atom,
the compound is water soluble only if it has 2 5 carbons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning