: 39% in 30m 20 CALENDAR Travel to Home Today at 11:02 PM Green Street f... Lumen... Sign In or School.. ohm Rukayat Balogun오 | User Settings My Classes Log Out ork manager ges Forums Calendar Gradebook 1:30am Fall 2018> Assessment pplications of Systems of Equations Due in 11 hours, 28 minutes. Due Fri 12/21/2018 10:00 am Monico needs to mix a 10% alcohol solution with a 50% alcohol solution to create 100 milli!eters of a 26% solution. How many millileters of each solution must Monico use? Answer: Monico must mix -milli!eters of 10% solution and milli!eters of 50% solution. Points possible: 1 Unlimited attempts License Submit on
: 39% in 30m 20 CALENDAR Travel to Home Today at 11:02 PM Green Street f... Lumen... Sign In or School.. ohm Rukayat Balogun오 | User Settings My Classes Log Out ork manager ges Forums Calendar Gradebook 1:30am Fall 2018> Assessment pplications of Systems of Equations Due in 11 hours, 28 minutes. Due Fri 12/21/2018 10:00 am Monico needs to mix a 10% alcohol solution with a 50% alcohol solution to create 100 milli!eters of a 26% solution. How many millileters of each solution must Monico use? Answer: Monico must mix -milli!eters of 10% solution and milli!eters of 50% solution. Points possible: 1 Unlimited attempts License Submit on
Linear Algebra: A Modern Introduction
4th Edition
ISBN:9781285463247
Author:David Poole
Publisher:David Poole
Chapter4: Eigenvalues And Eigenvectors
Section4.6: Applications And The Perron-frobenius Theorem
Problem 25EQ
Related questions
Question
Transcribed Image Text:: 39%
in 30m
20 CALENDAR
Travel to Home
Today at 11:02 PM
Green Street
f... Lumen... Sign In or
School..
ohm
Rukayat Balogun오 | User Settings My Classes
Log Out
ork manager
ges Forums Calendar Gradebook
1:30am Fall 2018> Assessment
pplications of Systems of Equations
Due in 11 hours, 28 minutes. Due Fri 12/21/2018 10:00 am
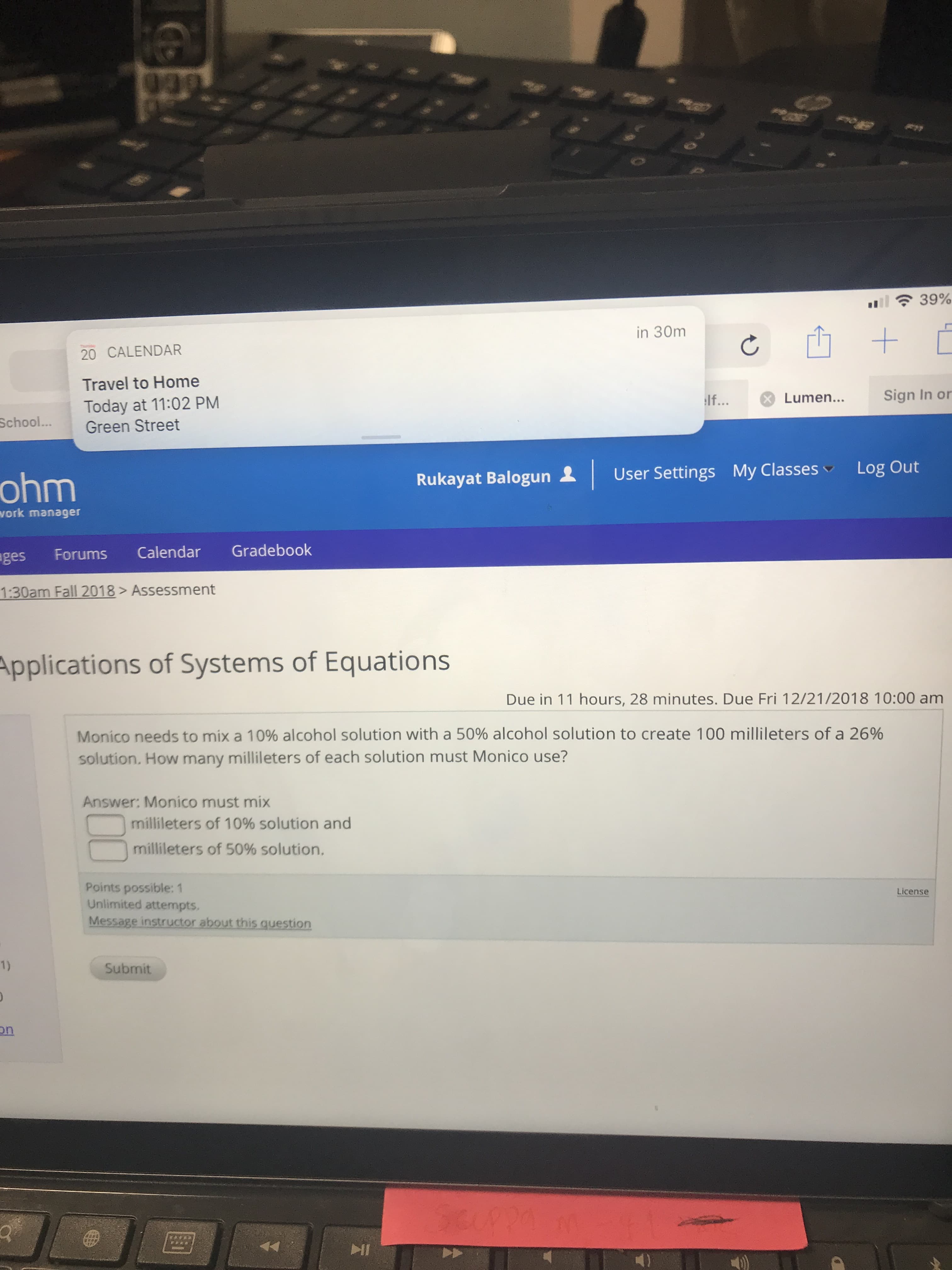
Monico needs to mix a 10% alcohol solution with a 50% alcohol solution to create 100 milli!eters of a 26%
solution. How many millileters of each solution must Monico use?
Answer: Monico must mix
-milli!eters of 10% solution and
milli!eters of 50% solution.
Points possible: 1
Unlimited attempts
License
Submit
on
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning