3C. Below are the gas chromatography results of two separate distillations of the same 1:1 mixture of ethanol and 1-butanol. One sample was obtained from the first fraction of a simple distillation and one sample was obtained from the first fraction of a fractional distillation. Identify the peaks in the chromatographs. Discuss the conclusions you can make regarding simple distillation compared with fractional distillation. Which method was more successful? Intensity (PA) Intensity (PA) 600000 500000 400000 300000 200000 100000 0 -100000 600000 500000 400000 300000 200000 100000 0 -100000 0.5 1 0.5 1 1 2 Simple Distillation GC 1.5 1 2 2 Simple Distillation Peak Number Retention Time (min) Peak Area %) 2.198 3.389 1.5 2.5 Fractional Distillation GC Time (min) 2 2.5 3 Time (min) 3.5 3 69.489 30.511 3.5 Fractional Distillation Peak Number Retention Time (min) Peak Area %) 2.226 95.381 3.513 4.619 4 4 4.5 4.5
3C. Below are the gas chromatography results of two separate distillations of the same 1:1 mixture of ethanol and 1-butanol. One sample was obtained from the first fraction of a simple distillation and one sample was obtained from the first fraction of a fractional distillation. Identify the peaks in the chromatographs. Discuss the conclusions you can make regarding simple distillation compared with fractional distillation. Which method was more successful? Intensity (PA) Intensity (PA) 600000 500000 400000 300000 200000 100000 0 -100000 600000 500000 400000 300000 200000 100000 0 -100000 0.5 1 0.5 1 1 2 Simple Distillation GC 1.5 1 2 2 Simple Distillation Peak Number Retention Time (min) Peak Area %) 2.198 3.389 1.5 2.5 Fractional Distillation GC Time (min) 2 2.5 3 Time (min) 3.5 3 69.489 30.511 3.5 Fractional Distillation Peak Number Retention Time (min) Peak Area %) 2.226 95.381 3.513 4.619 4 4 4.5 4.5
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter28: High-performance Liquid Chromatography
Section: Chapter Questions
Problem 28.7QAP
Related questions
Question
Below are the gas chromatography results of two separate distillations of the same 1:1 mixture of ethanol and 1-butanol. One sample was obtained from the first fraction of a simple distillation and one sample was obtained from the first fraction of a fractional distillation. Identify the peaks in the chromatographs. Discuss the conclusions you can make regarding simple distillation compared with fractional distillation. Which method was more successful?
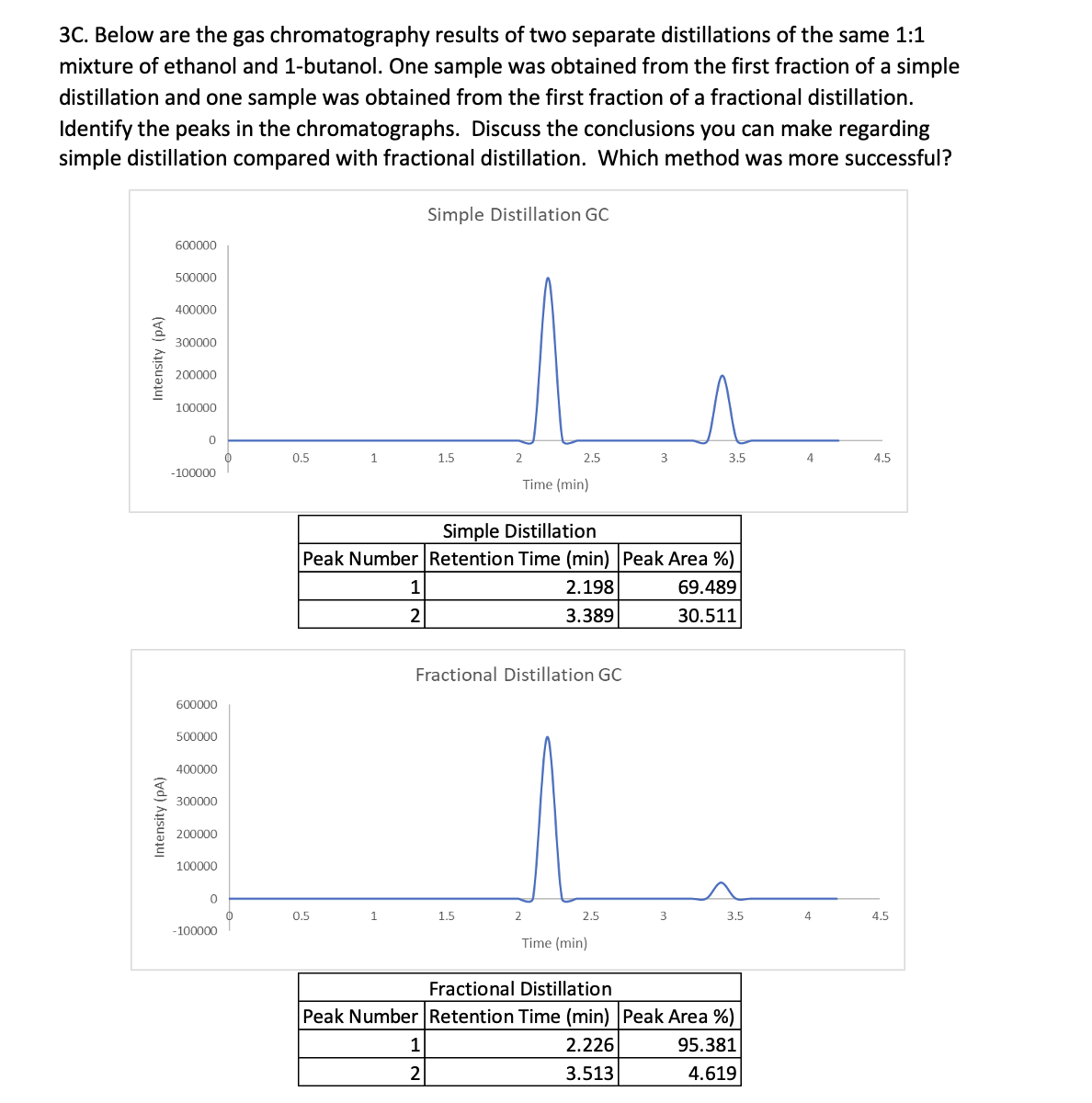
Transcribed Image Text:3C. Below are the gas chromatography results of two separate distillations of the same 1:1
mixture of ethanol and 1-butanol. One sample was obtained from the first fraction of a simple
distillation and one sample was obtained from the first fraction of a fractional distillation.
Identify the peaks in the chromatographs. Discuss the conclusions you can make regarding
simple distillation compared with fractional distillation. Which method was more successful?
Intensity (PA)
Intensity (PA)
600000
500000
400000
300000
200000
100000
0
-100000
600000
500000
400000
300000
200000
100000
0
-100000
0.5
1
Peak Number
1
2
0.5
1
Simple Distillation GC
1.5
1
2
2
1.5
2.5
Time (min)
Fractional Distillation GC
Simple Distillation
Retention Time (min) Peak Area %)
2.198
3.389
2
2.5
3
Time (min)
3.5
3
69.489
30.511
3.5
Fractional Distillation
Peak Number Retention Time (min) Peak Area %)
2.226
3.513
95.381
4.619
4
4
4.5
4.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning