4 An excited electron drops to its ground state, emitting a photon of frequency f = 1.58x1015 Hz. What is the energy of this photon. Enter the numerical value in units of eV?
4 An excited electron drops to its ground state, emitting a photon of frequency f = 1.58x1015 Hz. What is the energy of this photon. Enter the numerical value in units of eV?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.17QAP
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
2. u, kg
3. 1s2 2s2
Need help with #4
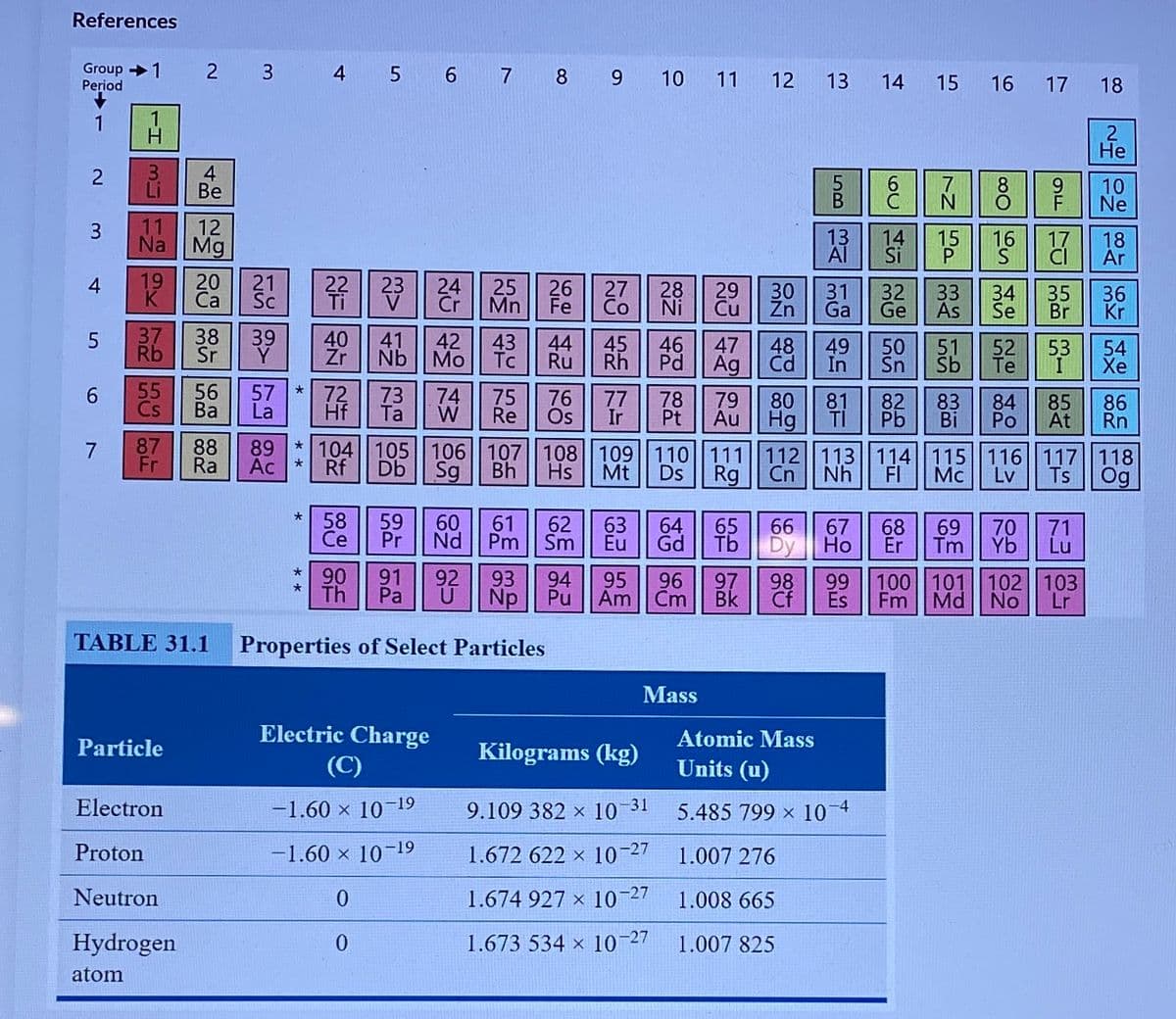
Transcribed Image Text:References
Group 1
Period
2 3
5 6 7 8 9
4
11
12
13
14
16
17
18
Не
4.
Li
10
Ne
Ве
3
11
12
13
14
Si
15
16
18
Ar
Na
Mg
Al
4
19
20
21
23
26
Cr Mn
27
28
29
30
Zn
31
32
34
33
Se
As
Ca
Sc
Co
Cu
35
36
Kr
Br
Fe
Ni
Ga
Ge
37
Rb
38
Sr
40
Źr
39
41
42
43
Tc
46
44
45
Rh
Pd
Ru
49
47
48
Cd
In
Ag
51
50
Sb
Sn
Nb
Мо
52
53
54
Te
I
Xe
55
56
57
La
72
Hf
73
Ta
6.
74
75
Re
76
Os
77
78
79
Cs
80
81
82
83
84
Po
85
At
86
Rn
Ва
Ir Pt AuHg 6 B
W
87
Fr
88
Ra
89
Ac
* | 104 105 | 106 107 108 109 110||111 112 113 114 115 116 117 118
Rf
7
Db
Sg
Bh
Hs
Mt
Ds
Rg | Cn | Nh
FI
Mc
Lv
Ts
Og
*
58
59
60
61
62
63
67 68
69
Tm
če|| Pr | Nd Pm Sm Eu Ğd Ťb by Ho Er
64
65
66
71
70
Yb
Lu
90
Th
91
Pa
92
93
Np
94
95
Am Cm
Pu
96
97
98
99
100 101
No
102 103
Md
Bk
Cf
Es
Fm
Lr
TABLE 31.1
Properties of Select Particles
Mass
Electric Charge
Atomic Mass
Particle
Kilograms (kg)
(C)
Units (u)
Electron
-1.60 × 10–19
9.109 382 × 10 31
5.485 799 x 10 4
Proton
-1.60 × 10-19
1.672 622 × 10-27
1.007 276
Neutron
1.674 927 x 10-27
1.008 665
Hydrogen
1.673 534 × 10-27
1.007 825
atom
15
10
2.
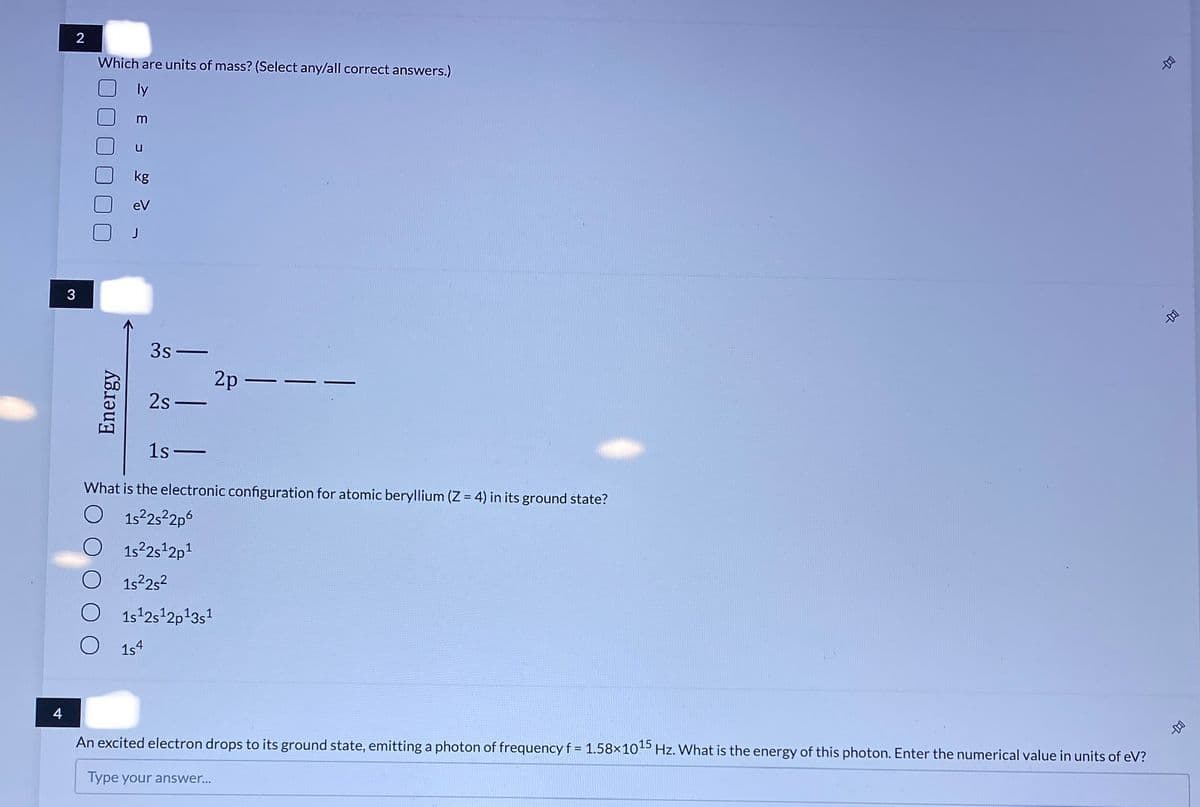
Transcribed Image Text:Which are units of mass? (Select any/all correct answers.)
m
kg
eV
3
3s-
2p –
2s -
1s-
What is the electronic configuration for atomic beryllium (Z = 4) in its ground state?
O 152252p6
1s2s 2p1
O 1s252
O 1s42s 2p13s
1s4
An excited electron drops to its ground state, emitting a photon of frequency f = 1.58×1045 Hz. What is the energy of this photon. Enter the numerical value in units of eV?
Type your answer...
Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning