(4 blanks) An ice-making machine operates in a Carnot cycle. It takes heat from water at 0.0 °C and rejects heat to a room at 24.0 °C. Suppose that 85.0 kg of water at 0.0 °C are converted to ice at 0.0 °C. How much energy (in Joules) must be supplied to the machine to achieve this task? Sol. Since the machine is a operating in a Carnot cycle, then, its efficiency is e = th |-| tc TH| By virtue of first law, the work done to convert water to ice is given by W = Q = ml Thus, the total energy needed to convert 85 kg of water is ET = 687.5 J
(4 blanks) An ice-making machine operates in a Carnot cycle. It takes heat from water at 0.0 °C and rejects heat to a room at 24.0 °C. Suppose that 85.0 kg of water at 0.0 °C are converted to ice at 0.0 °C. How much energy (in Joules) must be supplied to the machine to achieve this task? Sol. Since the machine is a operating in a Carnot cycle, then, its efficiency is e = th |-| tc TH| By virtue of first law, the work done to convert water to ice is given by W = Q = ml Thus, the total energy needed to convert 85 kg of water is ET = 687.5 J
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
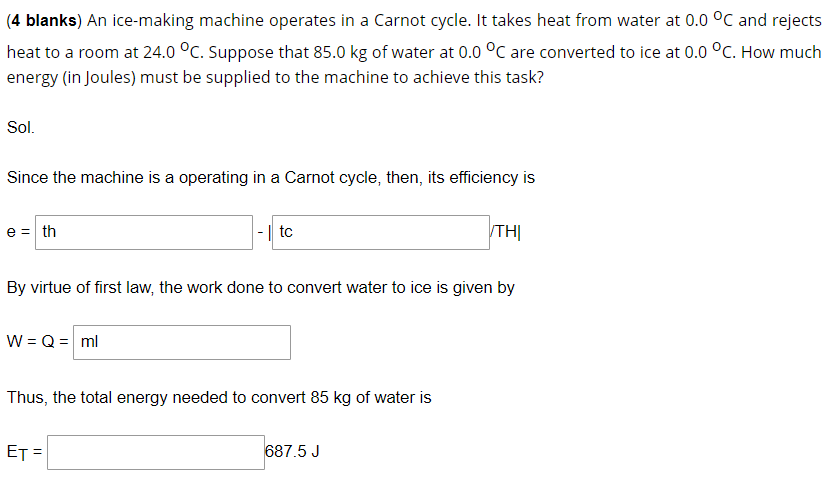
Transcribed Image Text:(4 blanks) An ice-making machine operates in a Carnot cycle. It takes heat from water at 0.0 °C and rejects
heat to a room at 24.0 °C. Suppose that 85.0 kg of water at 0.0 °C are converted to ice at 0.0 °C. How much
energy (in Joules) must be supplied to the machine to achieve this task?
Sol.
Since the machine is a operating in a Carnot cycle, then, its efficiency is
e = th
|-| tc
TH|
By virtue of first law, the work done to convert water to ice is given by
W = Q = ml
Thus, the total energy needed to convert 85 kg of water is
ET
687.5 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY