4) Environmental chemists mostly define solutions in molar units. Environmental engineers typically use mass units like mg/L. Convert the following concentrations: Solute mg/L NaCl 0.01 Na2CO3 10.0 NH3 1.0 x 106
4) Environmental chemists mostly define solutions in molar units. Environmental engineers typically use mass units like mg/L. Convert the following concentrations: Solute mg/L NaCl 0.01 Na2CO3 10.0 NH3 1.0 x 106
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.13: Bearly Alive: Solution Concentration
Problem 10E
Related questions
Question
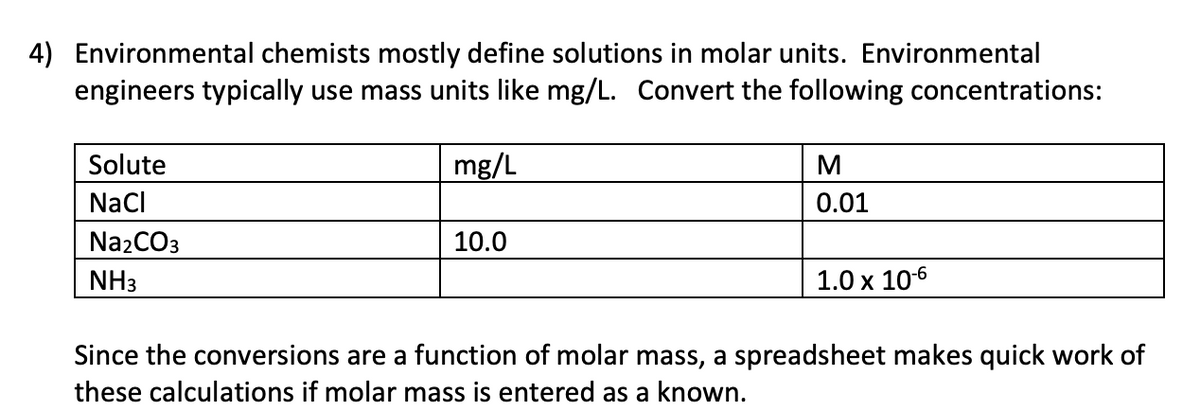
Transcribed Image Text:4) Environmental chemists mostly define solutions in molar units. Environmental
engineers typically use mass units like mg/L. Convert the following concentrations:
Solute
mg/L
Nacl
0.01
Na2CO3
10.0
NH3
1.0 x 106
Since the conversions are a function of molar mass, a spreadsheet makes quick work of
these calculations if molar mass is entered as a known.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
