4- In the antacid back titration lab, you need to use a standardized solution of NaOH to perform the back titration. Standardization is the process of determining the exact concentration of a given solution. NaOH is a secondary standard as it adsorbs water and reacts with CO2 to give NaHCO3. True False
4- In the antacid back titration lab, you need to use a standardized solution of NaOH to perform the back titration. Standardization is the process of determining the exact concentration of a given solution. NaOH is a secondary standard as it adsorbs water and reacts with CO2 to give NaHCO3. True False
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 13E: For a titration to be effective, the reaction must be rapid and the yield of the reaction must...
Related questions
Question
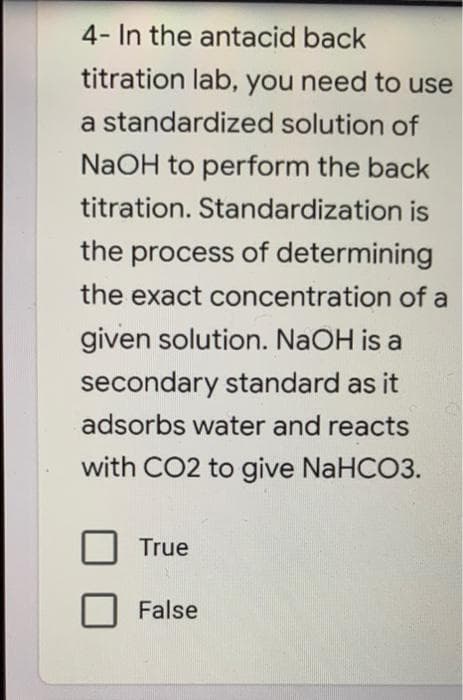
Transcribed Image Text:4- In the antacid back
titration lab, you need to use
a standardized solution of
NaOH to perform the back
titration. Standardization is
the process of determining
the exact concentration of a
given solution. NAOH is a
secondary standard as it
adsorbs water and reacts
with CO2 to give NaHCO3.
True
False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
