4) Propane, C3 Hg (g), is gaseous fuel that burns with oxygen. Write the balanced chemical equation for this complete combustion reaction, and use the bond energies from table 1 to calculate the quantity of energy released.
4) Propane, C3 Hg (g), is gaseous fuel that burns with oxygen. Write the balanced chemical equation for this complete combustion reaction, and use the bond energies from table 1 to calculate the quantity of energy released.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter9: Ionic And Covalent Bonding
Section: Chapter Questions
Problem 9.86QP: A commercial process for preparing ethanol (ethyl alcohol), C2H5OH, consists of passing ethylene...
Related questions
Question

Transcribed Image Text:4) Propane, C3 Hg (g), is gaseous fuel that burns with oxygen.
Write the balanced chemical equation for this complete
combustion reaction, and use the bond energies from table 1 to
calculate the quantity of energy released.
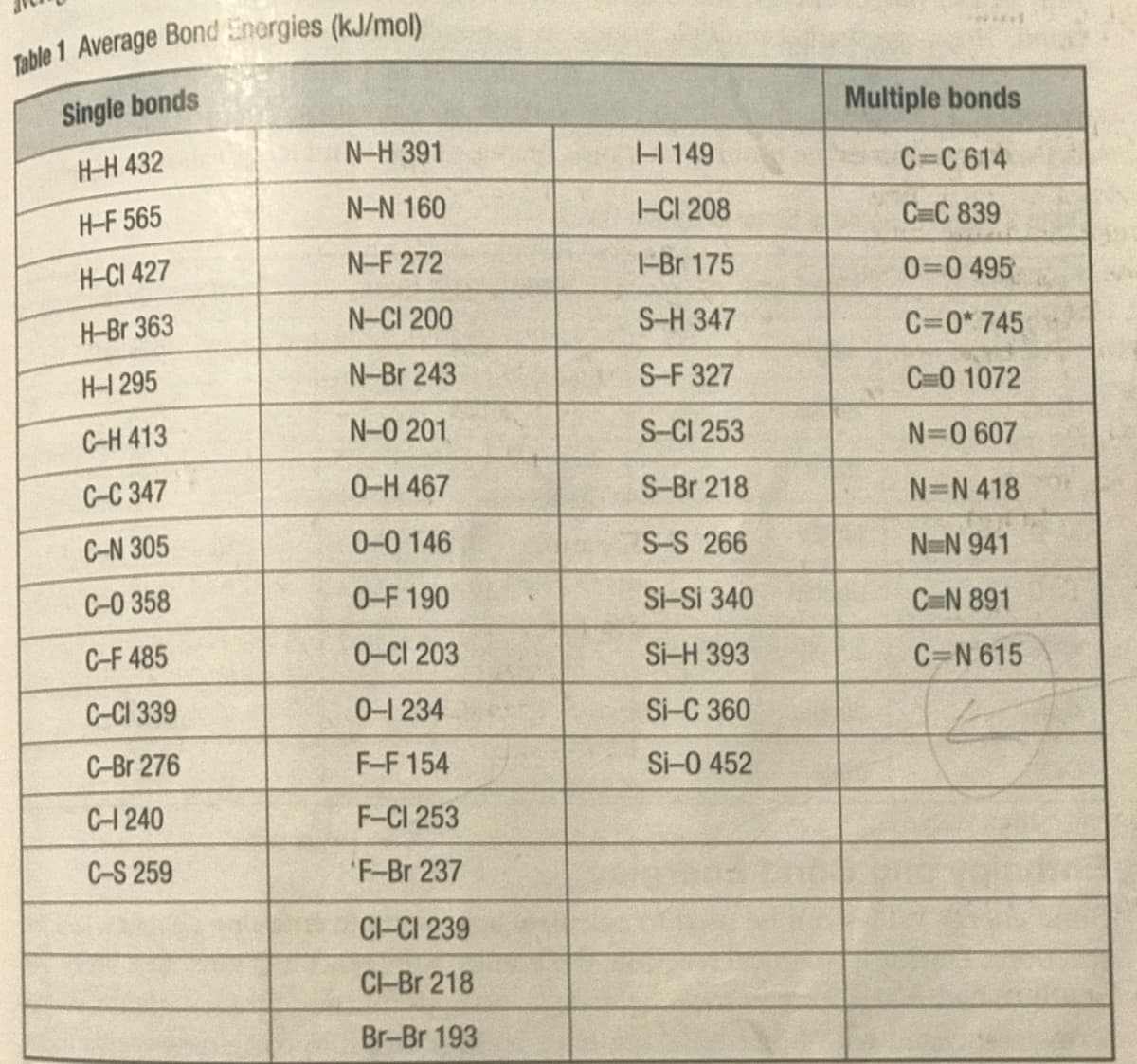
Transcribed Image Text:Single bonds
Multiple bonds
H-H 432
N-H 391
H149
C=C 614
H-F 565
N-N 160
-CI 208
C C 839
H-CI 427
N-F 272
-Br 175
0=0 495
H-Br 363
N-CI 200
S-H 347
C=0* 745
H-1 295
N-Br 243
S-F 327
C=0 1072
C-H 413
N-0 201
S-CI 253
N=0 607
C-C 347
0-H 467
S-Br 218
N=N 418
C-N 305
0-0 146
S-S 266
N-N 941
C-0 358
0-F 190
Si-Si 340
C=N 891
C-F 485
0-CI 203
Si-H 393
C=N 615
C-CI 339
0-1 234
Si-C 360
C-Br 276
F-F 154
Si-0 452
C-1240
F-CI 253
C-S 259
F-Br 237
CI-CI 239
CI-Br 218
Br-Br 193
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning