4 Which of the following ketones has the lowest boliling point? CH 3 to. 1 b. 4 C. 3 d. 2 5. What is the reason why carboxylic acids have higher boiling points than alcohols or aldehydes of similar molecular weight? to. Dispersion forces Hydrogen bonds C. The formation of the acid dimer 4 Dipole-dipole interactions 6. What is the name of the following compound? 10. Hexanal b 4-hexanol 3-hydroxyhexanal 4-hydroxyhexanal
4 Which of the following ketones has the lowest boliling point? CH 3 to. 1 b. 4 C. 3 d. 2 5. What is the reason why carboxylic acids have higher boiling points than alcohols or aldehydes of similar molecular weight? to. Dispersion forces Hydrogen bonds C. The formation of the acid dimer 4 Dipole-dipole interactions 6. What is the name of the following compound? 10. Hexanal b 4-hexanol 3-hydroxyhexanal 4-hydroxyhexanal
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter13: Alcohols, Phenols, And Ethers
Section: Chapter Questions
Problem 13.69E: Figure 13.13 focuses on the use of thiol chemistry by skunks. If thiols are more volatile than...
Related questions
Question
solve all please..
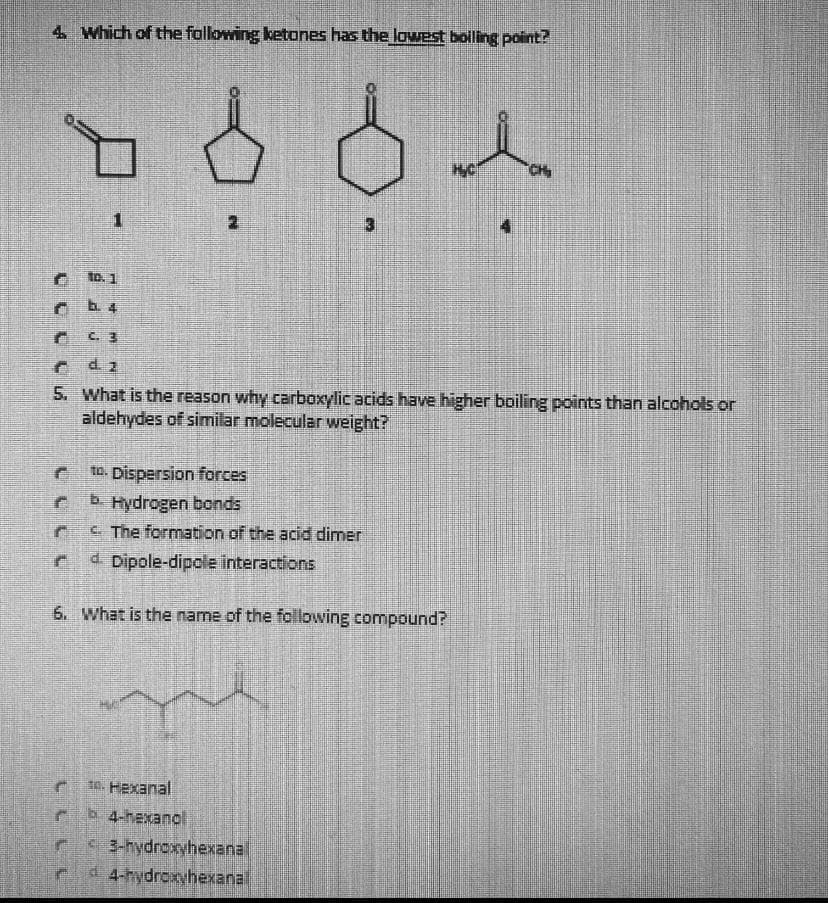
Transcribed Image Text:4. Which of the following ketones has the lowest boling point?
CH
to. 1
b. 4
C. 3
d. 2
5. What is the reason why carboxylic acids have higher boiling points than alcohols or
aldehydes of similar molecular weight?
to. Dispersion forces
Hydrogen bonds
C. The formation of the acid dimer
4 Dipole-dipole interactions
6. What is the name of the following compound?
t0. Hexanal
b 4-hexanol
3-hydroxyhexanal
4-hydroxyhexanal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax