4. (A) Indicate following statements as True (T) or False (F): (a) Higher the molar mass of a gas, faster its diffusion is. (b) Graham's law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. (c) When NH3(g) and HCI(g) react to form NH,CI(s), a blue precipitate is observed. ..... ........ .... (d) Diffua
4. (A) Indicate following statements as True (T) or False (F): (a) Higher the molar mass of a gas, faster its diffusion is. (b) Graham's law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. (c) When NH3(g) and HCI(g) react to form NH,CI(s), a blue precipitate is observed. ..... ........ .... (d) Diffua
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.114P: 5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous...
Related questions
Question
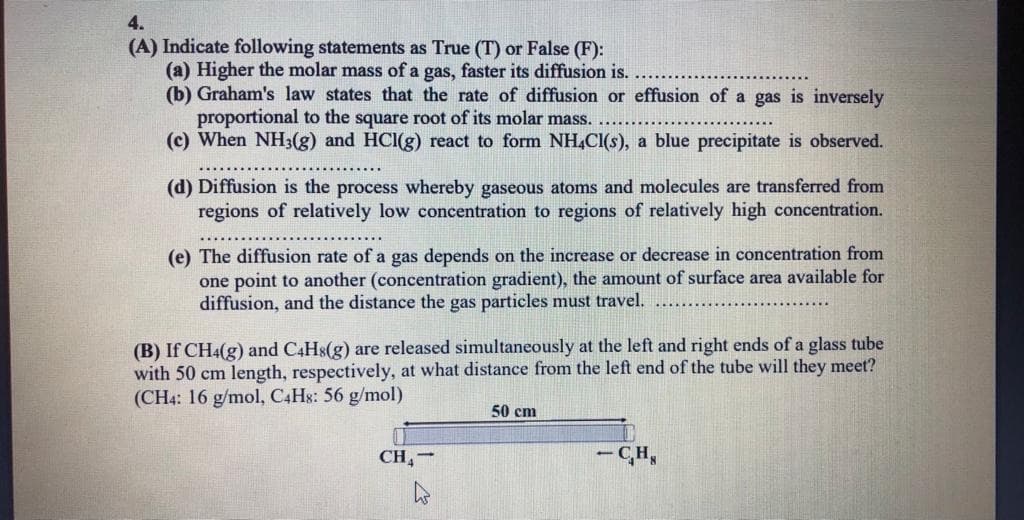
Transcribed Image Text:4.
(A) Indicate following statements as True (T) or False (F):
(a) Higher the molar mass of a gas, faster its diffusion is.
(b) Graham's law states that the rate of diffusion or effusion of a gas is inversely
proportional to the square root of its molar mass.
(c) When NH3(g) and HCI(g) react to form NH,Cl(s), a blue precipitate is observed.
(d) Diffusion is the process whereby gaseous atoms and molecules are transferred from
regions of relatively low concentration to regions of relatively high concentration.
(e) The diffusion rate of a gas depends on the increase or decrease in concentration from
one point to another (concentration gradient), the amount of surface area available for
diffusion, and the distance the gas particles must travel.
(B) If CH4(g) and C4HS(g) are released simultaneously at the left and right ends of a glass tube
with 50 cm length, respectively, at what distance from the left end of the tube will they meet?
(CH4: 16 g/mol, C4H8: 56 g/mol)
50 cm
CH,-
- CH,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning