4. A mass of 0.05 kg of carbon dioxide (molar mass 44 kg/kmol), occupying a volume of 0.03 m3 at 1.025 bar, is compressed reversibly until the pressure is 6.15bar. Calculate the final temperature, the work done on the CO2, and the heat supplied: i) 1.4 pv when the process is according to a law ii) when the process is isothermal iii) when the process takes place in a perfectly thermally insulated cylinder. Use Cp of CO2 as 939 J/kgK, which is the cp at 400K as per tables of properties page 17. = constant
4. A mass of 0.05 kg of carbon dioxide (molar mass 44 kg/kmol), occupying a volume of 0.03 m3 at 1.025 bar, is compressed reversibly until the pressure is 6.15bar. Calculate the final temperature, the work done on the CO2, and the heat supplied: i) 1.4 pv when the process is according to a law ii) when the process is isothermal iii) when the process takes place in a perfectly thermally insulated cylinder. Use Cp of CO2 as 939 J/kgK, which is the cp at 400K as per tables of properties page 17. = constant
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
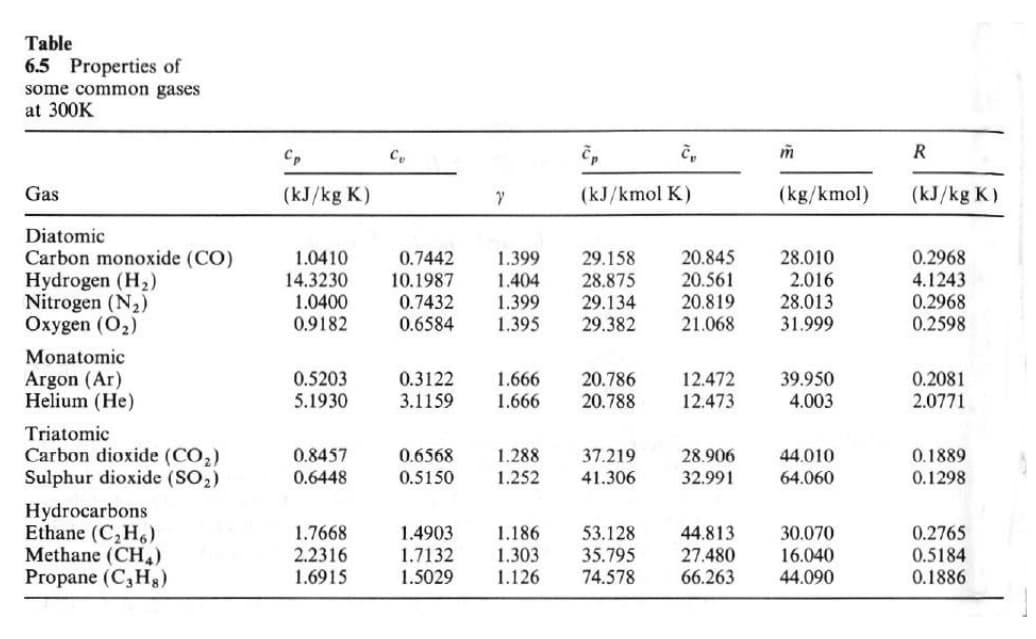
Transcribed Image Text:Table
6.5 Properties of
some common gases
at 300K
R
Gas
(kJ/kg K)
(kJ/kmol K)
(kg/kmol)
(kJ/kg K)
Diatomic
Carbon monoxide (CO)
Hydrogen (H2)
Nitrogen (N2)
Oxygen (O2)
0.2968
1.0410
14.3230
1.0400
0.9182
20.845
20.561
20.819
21.068
28.010
2.016
28.013
31.999
0.7442
1.399
29.158
28.875
29.134
29.382
4.1243
0.2968
0.2598
10.1987
1.404
1.399
1.395
0.7432
0.6584
Monatomic
Argon (Ar)
Helium (He)
0.5203
12.472
12.473
39.950
0.2081
2.0771
0.3122
1.666
1.666
20.786
20.788
5.1930
3.1159
4.003
Triatomic
Carbon dioxide (CO2)
Sulphur dioxide (SO2)
0.8457
0.6568
37.219
1.288
1.252
28.906
44.010
0.1889
0.6448
0.5150
41.306
32.991
64.060
0.1298
Hydrocarbons
Ethane (C,H6)
Methane (CH,)
Propane (C,Hg)
1.4903
1.7132
1.7668
1.186
53.128
35.795
74.578
44.813
27.480
66.263
30.070
0.2765
2.2316
1.6915
1.303
1.126
16.040
0.5184
0.1886
1.5029
44.090

Transcribed Image Text:4. A mass of 0.05 kg of carbon dioxide (molar mass 44 kg/kmol), occupying a volume of 0.03
m³ at 1.025 bar, is compressed reversibly until the pressure is 6.15bar. Calculate the final
temperature, the work done on the CO2, and the heat supplied:
i)
1.4
= constant
when the process is according to a law pv
ii) when the process is isothermal
iii) when the process takes place in a perfectly thermally insulated cylinder.
Use Cp of CO2 as 939 J/kgK, which is the cp at 400K as per tables of properties page 17.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY