4. Calculate the molarity of each of the following solutions: (a) 10.0 g H,SO, in 250 mL of solution (b) 6.00 g NaOH in 500 mL of solution (c) 25.0 g AGNO, in 1.00 L of solution 5. A 12.5 mL portion of a solution is diluted to 500 mL, and its molarity is determined to be 0.125. What is the molarity of the original solution? 6. How many milliliters of concentrated hydrochloric acid, 38% (wt/wt), specific gravity 1.19, are required to prepare 1 L of a 0.100 M solution? Assume density and specific gravity are equal within three significant figures.
4. Calculate the molarity of each of the following solutions: (a) 10.0 g H,SO, in 250 mL of solution (b) 6.00 g NaOH in 500 mL of solution (c) 25.0 g AGNO, in 1.00 L of solution 5. A 12.5 mL portion of a solution is diluted to 500 mL, and its molarity is determined to be 0.125. What is the molarity of the original solution? 6. How many milliliters of concentrated hydrochloric acid, 38% (wt/wt), specific gravity 1.19, are required to prepare 1 L of a 0.100 M solution? Assume density and specific gravity are equal within three significant figures.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.103P: 4-103 Caffeine, a central nervous system stimulant, has the molecular formula C8H10N402. (a) How...
Related questions
Question
Answer 4 5 6
Handwritten then box the final answer
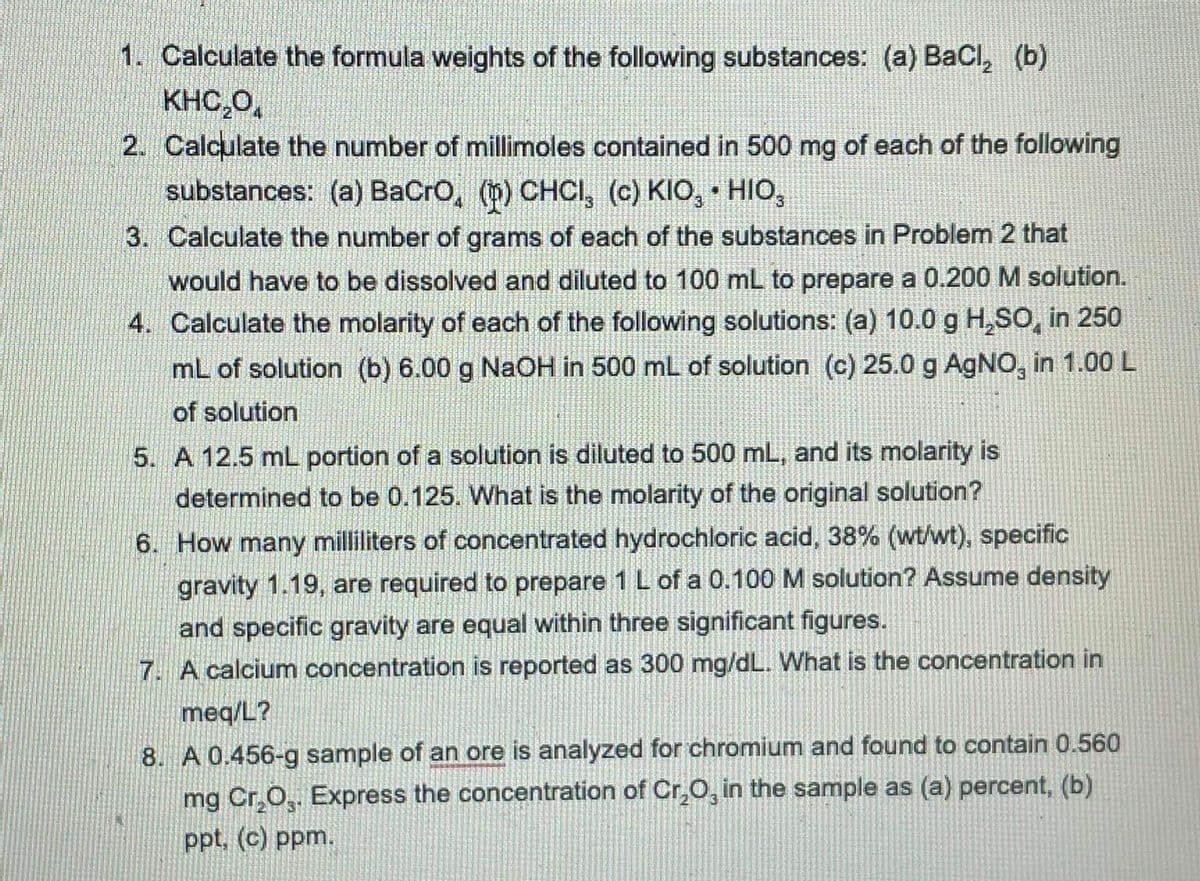
Transcribed Image Text:1. Calculate the formula weights of the following substances: (a) BaCl, (b)
KHC,0,
2. Calculate the number of millimoles contained in 500 mg of each of the following
substances: (a) BaCro, (b) CHCI, (c) KIO, • HIo,
3. Calculate the number of grams of each of the substances in Problem 2 that
would have to be dissolved and diluted to 100 mL to prepare a 0.200 M solution.
4. Calculate the molarity of each of the following solutions: (a) 10.0 g H,SO, in 250
mL of solution (b) 6.00 g NaOH in 500 mL of solution (c) 25.0 g AgNO, in 1.00 L
of solution
5. A 12.5 mL portion of a solution is diluted to 500 mL, and its molarity is
determined to be 0.125. What is the molarity of the original solution?
6. How many milliliters of concentrated hydrochloric acid, 38% (wt/wt), specific
gravity 1.19, are required to prepare 1 L of a 0.100 M solution? Assume density
and specific gravity are equal within three significant figures.
7. A calcium concentration is reported as 300 mg/dL. What is the concentration in
meq/L?
8. A 0.456-g sample of an ore is analyzed for chromium and found to contain 0.560
mg Cr,O,. Express the concentration of Cr,O, in the sample as (a) percent, (b)
ppt. (c) ppm.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning