4. Comment how the concentration affects the rate. a. Concentration definitely has an affect on the rate. When there is an increase in concentration, the rate as which the reaction occurs also increases. This is proved through my data. As concentration of oxalic acid increases, here are my rates that go from lowest concentration to highest concentration: 3*103 M/s, 6.8*10³ M/s, 9.26*10* M/s, 1.15*10“ M/s. Here, as the number of molecules in a given volume are increased (due to concentration increase) the number of effective collisions also increase. This causes the increase in reaction rate.
4. Comment how the concentration affects the rate. a. Concentration definitely has an affect on the rate. When there is an increase in concentration, the rate as which the reaction occurs also increases. This is proved through my data. As concentration of oxalic acid increases, here are my rates that go from lowest concentration to highest concentration: 3*103 M/s, 6.8*10³ M/s, 9.26*10* M/s, 1.15*10“ M/s. Here, as the number of molecules in a given volume are increased (due to concentration increase) the number of effective collisions also increase. This causes the increase in reaction rate.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 98CWP: A certain reaction has the form aAProducts At a particular temperature, concentration versus time...
Related questions
Question
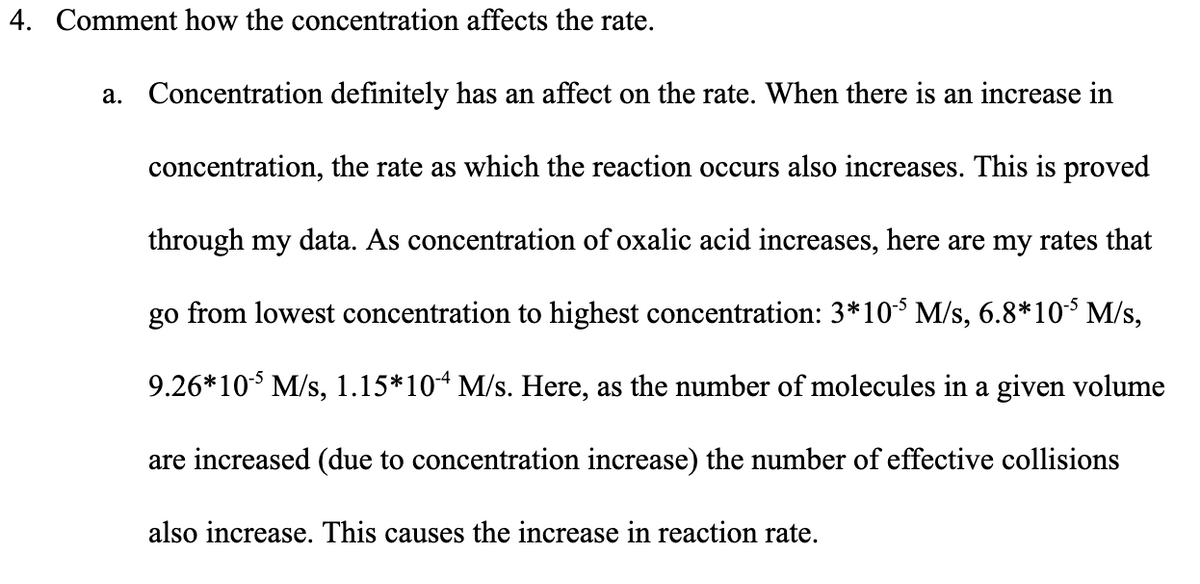
Transcribed Image Text:4. Comment how the concentration affects the rate.
a. Concentration definitely has an affect on the rate. When there is an increase in
concentration, the rate as which the reaction occurs also increases. This is proved
through my data. As concentration of oxalic acid increases, here are my rates that
go from lowest concentration to highest concentration: 3*10$ M/s, 6.8*10 M/s,
9.26*10 M/s, 1.15*104 M/s. Here, as the number of molecules in a given volume
are increased (due to concentration increase) the number of effective collisions
also increase. This causes the increase in reaction rate.
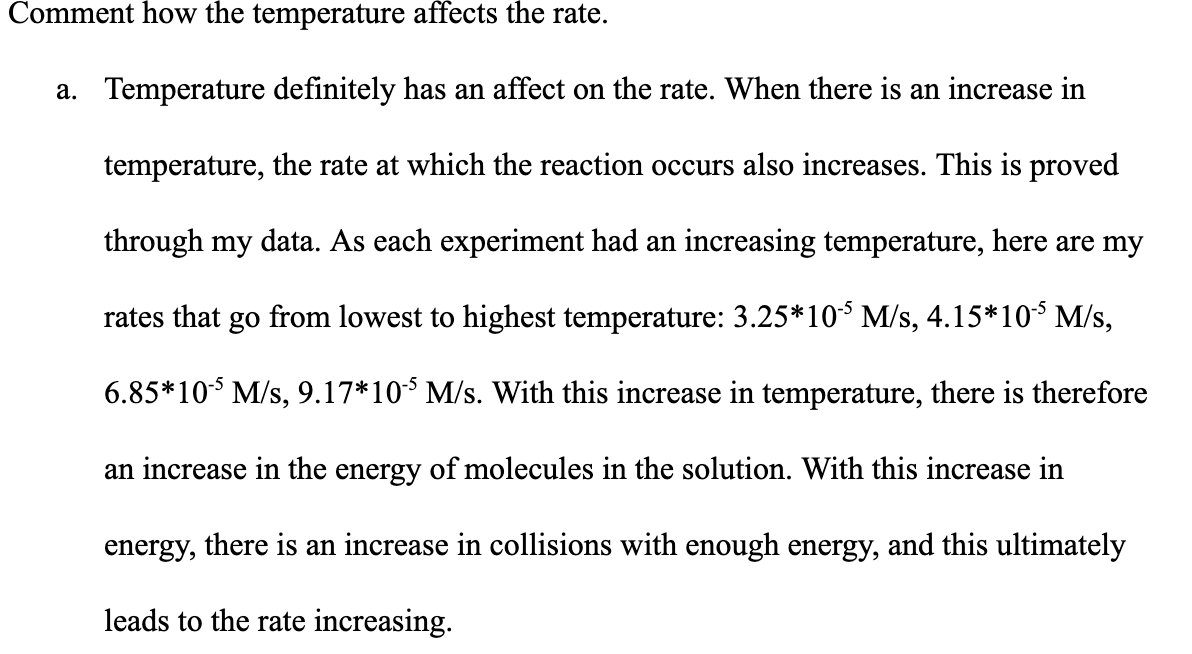
Transcribed Image Text:Comment how the temperature affects the rate.
a. Temperature definitely has an affect on the rate. When there is an increase in
temperature, the rate at which the reaction occurs also increases. This is proved
through my data. As each experiment had an increasing temperature, here are my
rates that go from lowest to highest temperature: 3.25*10 M/s, 4.15*10 M/s,
6.85*10 M/s, 9.17*10* M/s. With this increase in temperature, there is therefore
an increase in the energy of molecules in the solution. With this increase in
energy, there is an increase in collisions with enough energy, and this ultimately
leads to the rate increasing.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning