4. DETAILS SERCP11 10.4.OP.039. 0/2 Submissions Used MY NOTES ASK YOUR TEACHER An air bubble released by a remotely operated underwater vehicle, 120 m below the surface of a lake, has a volume of 1.35 cm. The surface of the lake is at sea level, and the density of the lake water can be approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm³) of the bubble just before it pops at the surface of the lake. cm3
4. DETAILS SERCP11 10.4.OP.039. 0/2 Submissions Used MY NOTES ASK YOUR TEACHER An air bubble released by a remotely operated underwater vehicle, 120 m below the surface of a lake, has a volume of 1.35 cm. The surface of the lake is at sea level, and the density of the lake water can be approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm³) of the bubble just before it pops at the surface of the lake. cm3
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 100P: One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a...
Related questions
Question
100%
#4 from this screenshot please.
Transcribed Image Text:O Files
Download Document
A Untitled document - Google X
A Topic 10 HW 1 (due 11:59p X
My Solutions | bartleby
A webassign.net/web/Student/Assignment-Responses/last?dep=25326192
Q
M
Update :
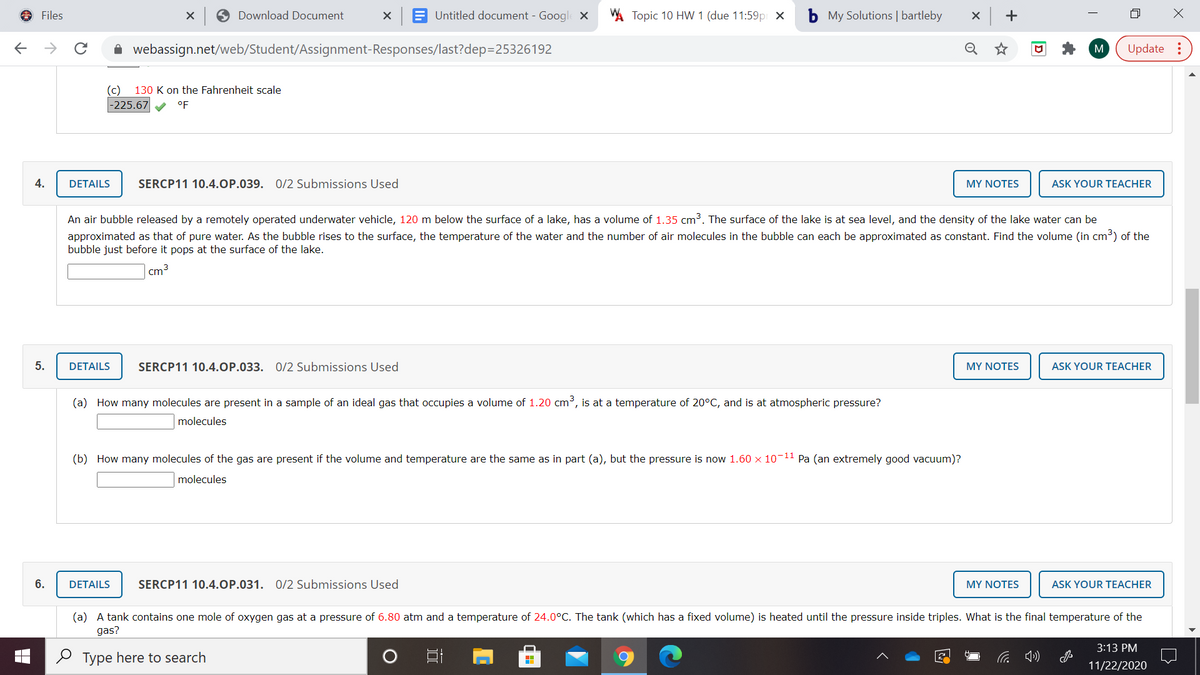
(c)
130 K on the Fahrenheit scale
-225.67
°F
4.
DETAILS
SERCP11 10.4.OP.039. 0/2 Submissions Used
MY NOTES
ASK YOUR TEACHER
An air bubble released by a remotely operated underwater vehicle, 120 m below the surface of a lake, has a volume of 1.35 cm³. The surface of the lake is at sea level, and the density of the lake water can be
approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm³) of the
bubble just before it pops at the surface of the lake.
cm 3
5.
DETAILS
SERCP11 10.4.0P.033. 0/2 Submissions Used
MY NOTES
ASK YOUR TEACHER
(a) How many molecules are present in a sample of an ideal gas that occupies a volume of 1.20 cm³, is at a temperature of 20°C, and is at atmospheric pressure?
molecules
(b) How many molecules of the gas are present if the volume and temperature are the same as in part (a), but the pressure is now 1.60 x 10-11 Pa (an extremely good vacuum)?
molecules
6.
DETAILS
SERCP11 10.4.0P.031. 0/2 Submissions Used
MY NOTES
ASK YOUR TEACHER
(a) A tank contains one mole of oxygen gas at a pressure of 6.80 atm and a temperature of 24.0°C. The tank (which has a fixed volume) is heated until the pressure inside triples. What is the final temperature of the
gas?
3:13 PM
2 Type here to search
11/22/2020
D
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning